Abstract
A main challenge in parasitology is the development of reliable tools to prevent or treat mosquito-borne diseases. We investigated the toxicity of magnetic nanoparticles (MNP) produced by Magnetospirillum gryphiswaldense (strain MSR-1) on chloroquine-resistant (CQ-r) and sensitive (CQ-s) Plasmodium falciparum, dengue virus (DEN-2), and two of their main vectors, Anopheles stephensi and Aedes aegypti, respectively. MNP were studied by Fourier-transform infrared spectroscopy and transmission electron microscopy. They were toxic to larvae and pupae of An. stephensi, LC50 ranged from 2.563 ppm (1st instar larva) to 6.430 ppm (pupa), and Ae. aegypti, LC50 ranged from 3.231 ppm (1st instar larva) to 7.545 ppm (pupa). MNP IC50 on P. falciparum were 83.32 μg ml−1 (CQ-s) and 87.47 μg ml−1 (CQ-r). However, the in vivo efficacy of MNP on Plasmodium berghei was low if compared to CQ-based treatments. Moderate cytotoxicity was detected on Vero cells post-treatment with MNP doses lower than 4 μg ml−1. MNP evaluated at 2–8 μg ml−1 inhibited DEN-2 replication inhibiting the expression of the envelope (E) protein. In conclusion, our findings represent the first report about the use of MNP in medical and veterinary entomology, proposing them as suitable materials to develop reliable tools to combat mosquito-borne diseases.
Similar content being viewed by others
Introduction
The very recent outbreaks of mosquito-borne diseases, such as chikungunya and Zika virus (Benelli and Mehlhorn 2016), highlighted the pivotal relevance of effective mosquito control programs. Nowadays, the management of Culicidae is challenging due to four main issues. First is the rapid development of mosquito resistance to synthetic molecules used as pesticides (Naqqash et al. 2016; Pavela and Benelli 2016). Second is the rapid development of resistance to the most commonly used antiplasmodial molecules, i.e., chloroquine and artemisinin (WHO 2015). Third is the spread of highly invasive mosquito vectors worldwide (Mehlhorn 2015). Fourth is the rise of new arboviruses extremely dangerous for public health, with special reference to marginalized populations, which do not have access to expensive vaccines or drugs and preventive tools (Benelli and Mehlhorn 2016). Therefore, the discovery of eco-friendly nano-pesticides to fight arthropod vectors is a key issue (Benelli 2015a, b, 2016a, b, c). A further hot challenge in parasitology is also the development of effective drugs to treat or prevent arthropod-borne diseases (Benelli et al. 2016a, b).
Magnetotactic bacteria, such as Magnetospirillum magnetotacticum and Magnetospirillum gryphiswaldense, are useful sources of magnetic nanoparticles (MNP), which are currently employed in medical and pharmacological sciences as well as in engineering and environmental science (Tartaj et al. 2003; Faraji et al. 2010). Peculiar finite-size and surface effects are mainly responsible for the magnetic behavior of these nanoparticles (Grancharov et al. 2005; Yoshino et al. 2010; Akbarzadeh et al. 2012). Recently, employing suspensions containing chains of magnetosomes has been suggested to treat cancers using magnetic hyperthermia (Alphandéry et al. 2011a, b, 2012, 2013). MNP usually showed a number of chemical groups on their surface, and this could be exploited for their bio-functionalization (Sun et al. 2011), achieving nanocomposites with high biocompatibility and low toxicity on non-target cells and tissues (Xiang et al. 2007; Scheffel et al. 2006).
In this scenario, to the best of our knowledge, the MNP synthesized by magnetotactic bacteria have been scarcely studied as potential sources of novel mosquitocides or antiplasmodial and anti-dengue drugs. Therefore, in the present work, we investigated the effectiveness of MNP produced by M. gryphiswaldense strain MSR-1 on CQ-resistant (CQ-r) and CQ-sensitive (CQ-s) Plasmodium falciparum, the serotype DEN-2 of dengue virus, and two of their main mosquito vectors, i.e., Anopheles stephensi and Aedes aegypti, respectively. Furthermore, after the purification process, MNP extracted from M. gryphiswaldense were studied by Fourier-transform infrared (FT-IR) spectroscopy and transmission electron microscopy (TEM) in order to shed light on their biophysical features.
Materials and methods
Extraction and purification of the nanoparticles
M. gryphiswaldense MSR-1 (DSM6361) cultures were carried out following the method by Liu et al. (2010). The identification, extraction, and purification of the MNP were conducted as recently described by Guo et al. (2011).
FT-IR and TEM characterization
For FT-IR and TEM assays, 30 μl (2 mg ml−1) of purified MNP were suspended in 30 μl of distilled water, then dried on a Zn disc and analyzed using FT-IR spectroscopy (Bruker, Vector 33), following Li et al. (2007a). Moreover, suspensions of M. gryphiswaldense cells and purified MNP in distilled water were adsorbed on a 300-mesh C-coated copper grid and analyzed by TEM (Philips Tecnai F 30).
Mosquito rearing and toxicity assays
An. stephensi and Ae. aegypti were reared following the method by Dinesh et al. (2015) and Suresh et al. (2015). MNP toxicity assays on larvae and pupae were conducted following Suresh et al. (2015). For each dose, five replicates were done (n = 25 mosquitoes per replicate). Control was dechlorinated water. Mortality (%) was noted 24 h post-treatment.
In vitro toxicity on P. falciparum
CQ-sensitive strain 3D7 and CQ-resistant strain INDO of P. falciparum were reared as described by Trager and Jensen (1976) with minor modifications by Murugan et al. (2015a). MNP formulated in DMSO were tested as described by Smilkstein et al. (2004) and Murugan et al. (2015a). IC50 were calculated. Results were confirmed by microscopical examination of Giemsa-stained smears of MNP-treated and control P. falciparum (Bagavan et al. 2011).
In vivo toxicity on Plasmodium berghei
P. berghei was cultured as described by Murugan et al. (2016a). MNP were tested on P. berghei following the 4-day suppressive method by Peters et al. (1975) with minor modifications by Ishih et al. (2003) and Murugan et al. (2016a); 4 days after the infection, the parasitemia of each mouse was calculated by microscopic examination (Ene et al. 2008). Chemosuppression (%) of the total parasitemia for each MNP dose was estimated as reported by Argotte et al. (2006).
Toxicity on DEN-2 virus
C6/36, Vero cells, and DEN-2 (New Guinea C strain) were cultured as described by Sujitha et al. (2015). DEN-2 inhibition assays were carried out following Murugan et al. (2015b). MNP were tested at 4, 6, and 8 μg ml−1 (five replicates per dose). DEN-2 yield was estimated by the plaque assay on Vero cells, monitoring the number of plaque-forming units (PFU) (Sujitha et al. 2015). Control was DEN-2-infected cells incubated in MNP-free medium for 36 h from the DEN-2 infection (Murugan et al. 2015b).
Furthermore, following Murugan et al. (2015b), Western blot analysis was employed to shed light on the effect of MNP on DEN-2 E protein expression. MNP were tested at 2, 4, 6, and 8 μg ml−1. Blots were incubated in TBS (TBST) mouse monoclonal anti-E antibody (1: 1000) in 0.1 % Tween 20. E expression was monitored by Western blot analysis, as reported by Murugan et al. (2015b).
Data analysis
Vector toxicity data were analyzed by probit analysis (Finney 1971) using the SPSS software 16.0. Plasmodium IC50 were calculated from drug concentration-response curves. DEN-2 PFU and E protein expression data were analyzed by ANOVA followed by Tukey’s HSD test (P = 0.05).
Results and discussion
Magnetic nanoparticle characterization
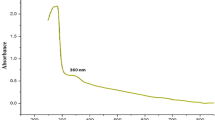
TEM of the MNP extracted from M. gryphiswaldense are given in Fig. 1. The membrane capping Fe3O4 granules (indicated by an arrow) indicated that the purification procedure reported here did not damage the bilayer of phospholipids characterizing the magnetosomes. To shed light on the purity of MNP isolated by the procedure reported here, the FT-IR spectrum was compared with one of the MNP isolated by Li et al. (2007a). We highlighted the absence of signals at 3273 and 2921 cm−1. This may be linked to NH bending and CH stretching of bound proteins (Fig. 2). Notably, the signals observed at 1728, 2355, and 2924 cm−1 could be due to NH and CH bending and disappearing of stretching modes (see also Mukunthan et al. 2011).
Toxicity on mosquito vectors
Recent reports pointed out the reduced health risk of MNP for human health (Li et al. 2007a, b). On this basis, it has been highlighted that they represent potential candidates for gene and drug delivery, for instance in cancer therapy (Sun et al. 2007, 2008). Indeed, suspensions of magnetosome chains have been successfully used by Alphandéry et al. (2011a, b, 2012, 2013) to treat cancers via magnetic hyperthermia. Notably, despite these interesting reports on the biological activities of MNP, to the best of our knowledge, no efforts have been carried out to test the effectiveness of MNP as mosquito pesticides or even as potential toxic agents against the parasites and pathogens vectored by arthropods.
The results presented in our research firstly showed that MNP were extremely toxic to An. stephensi and Ae. aegypti young instars (Tables 1 and 2). An. stephensi LC50 were 2.563 ppm (1st instar larvae), 3.203 ppm (2nd), 3.907 ppm (3rd), 5.048 ppm (4th), and 6.430 ppm (pupae). Ae. aegypti LC50 were 3.231 ppm (1st), 3.942 ppm (2nd), 4.845 (3rd), 5.959 (4th), and 7.545 ppm (pupae). Recent evidences underlined that nano-biopesticides can represent an important tool to boost the efficacy of mosquito control programs. For instance, it is noteworthy that an extensive number of metal, metal oxide, and carbon nanoparticles fabricated using botanicals or microorganisms are also effective to control Culicidae populations (e.g., Suresh et al. 2015; Govindarajan and Benelli 2016a, b; Jaganathan et al. 2016; Panneerselvam et al. 2016; Subramaniam et al. 2015, 2016). As a general trend, their LC50 values ranged between 10 and 30 ppm (Benelli 2016a), and only few of them showed LC50 values lower than 5 ppm (Benelli 2016c). Therefore, the toxicity results achieved by the MNP tested here on Anopheles and Aedes mosquito larvae can be considered as extremely promising. Further research on the mechanisms of action of MNP against mosquitoes, as well as on non-target aquatic organisms, is warranted.
Toxicity on P. falciparum and P. berghei
MNP IC50 calculated on P. falciparum were 83.32 μg ml−1 (CQ-s) and 87.47 μg ml−1 (CQ-r). On the other hand, the CQ IC50 were 92 μg ml−1 (CQ-s) and 96 μg ml−1 (CQ-r) (Fig. 3). In agreement with the present work, Murugan et al. (2015a) recently pointed out the high antiplasmodial activity of Ag nanoparticles fabricated using the aqueous extract of seaweed Ulva lactuca on P. falciparum strains. IC50 values were 76.33 μg ml−1 (CQ-s) and 79.13 μg ml−1 (CQ-r). Murugan et al. (2016a) noted that neem seed kernel-synthesized Ag nanoparticles achieved comparable IC50 on P. falciparum, with IC50 values of 82.41 μg ml−1 (CQ-s) and 86.12 μg ml−1 (CQ-r). As recently summarized by Benelli (2016c), the antiplasmodial activity of the abovementioned nanoformulations may be due to the inhibition of Plasmodium merozoite invasion into erythrocytes. Further studies on this issue are currently ongoing. Furthermore, Peters’ 4-day chemosuppressive activity assay showed a dose-dependent chemosuppression on P. berghei (Table 3). After 4 days from the MNP treatment, the parasitemia (%) of the test groups ranged from 7 ± 1.17 to 46.2 ± 2.32 %. On the other hand, with CQ administered at 5 mg kg−1 day−1, the mean parasitemia dropped to 1.0 ± 0.00 %, highlighting a higher efficacy, if compared to MNP (see also Rajakumar et al. 2015). Thus, in agreement with Murugan et al. (2016a), this latter result pointed out the key importance of in vivo tests, which should always follow in vitro screenings, since in vivo effectiveness on internal parasites often strongly differs from promising in vitro results (Mehlhorn 2015).
Toxicity on dengue virus (serotype DEN-2)
The present research reported moderate cytotoxicity rates on Vero cells exposed to MNP at concentrations low than 4 μg ml−1. Indeed, after the treatment, less than 20 % of the treated cells showed no viability (Fig. 4). Furthermore, MNP tested at 2–8 μg ml−1 significantly inhibit DEN-2 replication, with a reduction of the PFU abundance (Fig. 5). E protein expression trials evidenced that MNP blocked DEN-2 replication via inhibition of E expression (Fig. 6). Supporting our data, recent studies underlined the potential of metal nanoparticles as DEN-2 growth inhibitors (Sujitha et al. 2015). Ag nanoparticles down-regulate the expression of DEN-2 E gene (Murugan et al. 2015b). On the other hand, medium cytotoxicity of the tested Ag nanoparticles has been reported (e.g., 50 μg ml−1 led to a reduction of 30 % in cell viability). Then, Centroceras clavulatum-fabricated Ag nanoparticles tested at 50 μg ml−1 failed to show substantial cytotoxicity, while these inhibited the growth of DEN-2 of 80 % (Murugan et al. 2016b). Again, the findings discussed above underlined the key potential of screening an extensive number of botanical and microbial resources for parasitological purposes (Benelli 2016a; Benelli and Mehlhorn 2016).
(a) Inhibitory effect of magnetic nanoparticles isolated from Magnetospirillum gryphiswaldense on dengue (DEN-2) envelope (E) protein. (b) Relative expression of DEN-2 E protein/β-actin post-treatment with magnetic nanoparticles. T-bars represent standard errors. Above each column, different letters indicate significant differences (ANOVA, Tukey’s HSD test, P < 0.05)
Conclusions
In conclusion, the present research firstly showed that MNP isolated from M. gryphiswaldense were highly toxic to young instars of the mosquito vectors An. stephensi and Ae. aegypti. Furthermore, the MNP isolated here showed high efficacy on CQ-r P. falciparum. On the other hand, their in vivo efficacy on P. berghei was moderate if compared to that of CQ. Notably, moderate cytotoxicity was reported using doses lower than 4 μg ml−1, while 2–8 μg ml−1 of MNP were able to block the DEN-2 replication inhibiting E protein expression. The present work adds knowledge about the use of MNP in entomology and parasitology, allowing us to propose MNP isolated from M. gryphiswaldense as a rapid and reliable strategy for mosquito control as well as for the development of drugs to combat dengue and other arboviral diseases.
References
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144–157
Alphandéry E, Faure S, Raison L, Duguet E, Howse PA, Bazylinski DA (2011a) Heat production by bacterial magnetosomes exposed to an oscillating magnetic field. J Phys Chem C 115:18–22
Alphandéry E, Faure S, Seksek O, Guyot F, Chebbi I (2011b) Chains of magnetosomes extracted from AMB-1magnetotactic bacteria for application in alternative magnetic field cancer therapy. ACS Nano 5:6279–6296
Alphandéry E, Guyot F, Chebbi I (2012) Preparation of chains of magnetosomes isolated from Magnetospirillum magneticum strainAMB-1 magnetotactic bacteria, yielding efficient treatment of tumors using magnetic hyperthermia. Int J Pharm 434:444–452
Alphandéry E, Chebbi I, Guyot F, Durand-Dubief M (2013) Use of bacterial magnetosomes in the magnetic hyperthermia treatment of tumours: a review. Int J Hypertherm 29:801–809
Argotte RR, Ramírez AG, Rodríguez GMC et al (2006) Antimalarial 4-phenylcoumarins from the stem bark of Hintonia latiflora. J Nat Prod 69:1442–1444
Bagavan A, Rahuman AA, Kaushik NK, Sahal D (2011) In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol Res 108:15–22
Benelli G (2015a) Research in mosquito control: current challenges for a brighter future. Parasitol Res 114:2801–2805
Benelli G (2015b) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114:3201–3212
Benelli G (2016a) Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer—a brief review. Enzyme Microbial Technol doi: 10.1016/j.enzmictec.2016.08.022
Benelli G (2016a) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res 115:23–34
Benelli G (2016b) Plant-mediated synthesis of nanoparticles: a newer and safer tool against mosquito-borne diseases? Asia Pacif J Trop Biomed 6:353–354
Benelli G, Mehlhorn H (2016) Declining malaria, rising dengue and Zika virus: insights for mosquito vector control. Parasitol Res 115:1747–54
Benelli G, Canale A, Higuchi A, Murugan K, Pavela R, Nicoletti M (2016a) The outbreaks of Zika virus: mosquito control faces a further challenge. Asia Pacif J Trop Dis 6:253–258
Benelli G, Lo Iacono A, Canale A, Mehlhorn H (2016b) Mosquito vectors and the spread of cancer: an overlooked connection? Parasitol Res 115:2131–2137
Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U (2015) Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res 114:1519–1529
Ene AC, Atawodi SE, Ameh DA, Kwanshie HO, Agomo PU (2008) Experimental induction of chloroquine resistance in Plasmodium berghei NK 65. Tr Med Res 3(1):16–23
Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization and applications. J Iran Chem Soc 7:1–37
Finney DJ (1971) Probit analysis. Cambridge University, London 68–78
Govindarajan M, Benelli G (2016a) Facile biosynthesis of silver nanoparticles using Barleria cristata: mosquitocidal potential and biotoxicity on three non-target aquatic organisms. Parasitol Res. doi:10.1007/s00436-015-4817-0
Govindarajan M, Benelli G (2016b) One-pot green synthesis of silver nanocrystals using Hymenodictyon orixense: a cheap and effective tool against malaria, chikungunya and Japanese encephalitis mosquito vectors? RSC Adv 6:59021–59029
Grancharov SG, Zeng H, Sun SH, Wang SX, O’Brien S (2005) Biofunctionalization of monodisperse magnetic nanoparticles and their use as biomolecular labels in a magnetic tunnel junction based sensor. J Phys Chem B 109(26):13030–13035
Guo FF, Liu Y, Cheng YP, Tang T, Jiang W, Li Y, Li J (2011) A novel rapid and continuous procedure for large-scale purification of magnetosomes from Magnetospirillum gryphiswaldense. Appl Microbiol Biotechnol 90:1277–1283
Ishih A, Suzuki T, Watanabe M, Miyase T, Terada M (2003) Combination effects of chloroquine with the febrifugine and isofebrifugine mixture against a blood-induced infected with chloroquine-resistant Plasmodium berghei NK65 in ICR mice. Phytotherapy Res 17:1234–6
Jaganathan A, Murugan K, Panneerselvam C, Madhiyazhagan P, Dinesh D, Vadivalagan C, Aziz AT, Chandramohan B, Suresh U, Rajaganesh R, Subramaniam J, Nicoletti M, Higuchi A, Alarfaj AA, Munusamy MA, Kumar S, Benelli G (2016) Earthworm-mediated synthesis of silver nanoparticles: a potent tool against hepatocellular carcinoma, pathogenic bacteria, Plasmodium parasites and malaria mosquitoes. Parasitol Int 65:276–284
Li X, Jiang W, Sun JB, Wang GL, Guan F, Li Y (2007a) Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett Appl Microbiol 45(1):75–81
Li X, Jiang W, Sun JB, Wang GL, Guan F, Li Y (2007b) Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett Appl Microbiol 45(1):75–81
Liu Y, Li RG, Guo FF, Jiang W, Li Y, Li J (2010) Large-scale production of magnetosomes by chemostat culture of Magnetospirillum gryphiswaldense at high cell density. Microb Cell Fact 9:99
Mehlhorn H (ed) (2015) Encyclopedia of parasitology, 4th edn. Springer, New York, 893
Mukunthan KS, Elumalai EK, Patel TN, Murty VR (2011) Catharanthus roseus: a natural source for the synthesis of silver nanoparticles. Asian Pac J Trop Biomed 1:270–274
Murugan K, Samidoss CM, Panneerselvam C, Higuchi A, Roni M, Suresh U, Chandramohan B, Subramaniam J, Madhiyazhagan P, Dinesh D, Rajaganesh R, Alarfaj AA, Nicoletti M, Kumar S, Wei H, Canale A, Mehlhorn H, Benelli G (2015a) Seaweed-synthesized silver nanoparticles: an eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol Res 114:4087–4097
Murugan K, Dinesh D, Paulpandi M, Althbyani AD, Subramaniam J, Madhiyazhagan P, Wang L, Suresh U, Kumar PM, Mohan J, Rajaganesh R, Wei H, Kalimuthu K, Parajulee MN, Mehlhorn H, Benelli G (2015b) Nanoparticles in the fight against mosquito-borne diseases: bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae). Parasitol Res 114:4349–61
Murugan K, Panneerselvam C, Samidoss CM, Madhiyazhagan P, Suresh U, Roni M, Chandramohan B, Subramaniam J, Dinesh D, Rajaganesh R, Paulpandi M, Wei H, Aziz AT, Saleh Alsalhi M, Devanesan S, Nicoletti M, Pavela R, Canale A, Benelli G (2016a) In vivo and in vitro effectiveness of Azadirachta indica-synthesized silver nanocrystals against Plasmodium berghei and Plasmodium falciparum, and their potential against malaria mosquitoes. Res Vet Sci 106:14–22
Murugan K, Aruna P, Panneerselvam C, Madhiyazhagan P, Paulpandi M, Subramaniam J, Rajaganesh R, Wei H, Alsalhi MS, Devanesan S, Nicoletti M, Syuhei B, Canale A, Benelli G (2016b) Fighting arboviral diseases: low toxicity on mammalian cells, dengue growth inhibition (in vitro) and mosquitocidal activity of Centroceras clavulatum-synthesized silver nanoparticles. Parasitol Res 115:651–662
Naqqash MN, Gökçe A, Bakhsh A, Salim M (2016) Insecticide resistance and its molecular basis in urban insect pests. Parasitol Res 115:1363–1373
Panneerselvam C, Murugan K, Roni M, Aziz AT, Suresh U, Rajaganesh R, Madhiyazhagan P, Subramaniam J, Dinesh D, Nicoletti M, Higuchi A, Alarfaj AA, Munusamy MA, Kumar S, Desneux N, Benelli G (2016) Fern-synthesized nanoparticles in the fight against malaria: LC/MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitol Res 115:997–1013
Pavela R, Benelli G (2016) Essential oils as eco-friendly biopesticides? Challenges and constraints. Tr Plant Sci, doi: 10.1016/j.tplants.2016.10.005
Peters W, Portus JH, Robinson BL (1975) The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol 69:155–71
Rajakumar G, Rahuman AA, Chung IM, Vishnu Kirthi A, Marimuthu S, Anbarasan K (2015) Antiplasmodial activity of eco-friendly synthesized palladium nanoparticles using Eclipta prostrata extract against Plasmodium berghei in Swiss albino mice. Parasitol Res 114:1397–1406
Scheffel A, Gruska M, Faivre D, Linaroudis A, Graumann PL (2006) An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 440:110–14
Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high-through put antimalarial drug screening. Anti Microb Agents Chemother 48(5):1803–6
Subramaniam J, Murugan K, Panneerselvam C, Kovendan K, Madhiyazhagan P, Kumar PM, Dinesh D, Chandramohan B, Suresh U, Nicoletti M, Higuchi A, Hwang JS, Kumar S, Alarfaj AA, Munusamy MA, Messing RH, Benelli G (2015) Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ Sci Pollut Res Int 22:20067–20083
Subramaniam J, Murugan K, Panneerselvam C, Kovendan K, Madhiyazhagan P, Dinesh D, Mahesh Kumar P, Chandramohan B, Suresh U, Rajaganesh R, Saleh Alsalhi M, Devanesan S, Nicoletti M, Canale A, Benelli G (2016) Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: high antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ Sci Poll Res 23:7543–7558
Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, Nicoletti M, Higuchi A, Madhiyazhagan P, Subramaniam J, Dinesh D, Vadivalagan C, Chandramohan B, Alarfaj AA, Munusamy MA, Barnard DR, Benelli G (2015) Green synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol Res 114:3315–3325
Sun JB, Duan JH, Dai SL, Ren J, Zhang YD, Tian JS, Li Y (2007) In vitro and in vivo antitumor effects of doxorubicin loaded with bacterial magnetosomes (DBMs) on H22 cells: the magnetic bionanoparticles as drug carriers. Cancer Lett 258:109–117
Sun JB, Duan JH, Dai SL, Ren J, Guo L (2008) Preparation and antitumor efficiency evaluation of doxorubicin-loaded bacterial magnetosomes: magnetic nanoparticles as drug carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol Bioeng 101:1313–1320
Sun J, Li Y, Liang, XJ, Wang PC (2011) Bacterial magnetosome: a novel biogenetic magnetic targeted drug carrier with potential multifunctions. J Nano Mat 2011:469031 doi: 101155/2011/469031
Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C, Mahesh Kumar P, Subramaniam J, Dinesh D, Chandramohan B (2015) Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol Res 114:1551–1562
Tartaj P, Morales MDD, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Serna CJ (2003) The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D: Appl Phys 36:182–197
Trager W, Jensen J (1976) Human malaria parasites in continuous culture. Science 193:673–675
WHO (2015) WHO updates on artemisinin resistance. http://www.who.int/malaria/areas/drug_resistance/updates/en/
Xiang L, Wei J, Jianbo S, Gulli W, Feng G, Ying L (2007) Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett Appl Microbiol 45:75–81
Yoshino T, Maeda Y, Matsunaga T (2010) Bioengineering of bacterial magnetic particles and their applications in biotechnology. Rec Pat Biotechnol 4:214–225
Acknowledgments
The authors would like to thank Prof. H. Mehlhorn and the anonymous reviewers for their helpful suggestions on an earlier version of this study. Furthermore, the authors are grateful to the Deanship of Scientific Research, King Saud University, Saudi Arabia, for the financial support (project no. RGP-1435-057). G. Benelli is sponsored by PROAPI (PRAF 2015) “Valutazione della qualità organolettica del polline d’api fresco sottoposto a differenti trattamenti di condizionamento” and University of Pisa, Department of Agriculture, Food and Environment (Grant ID: COFIN2015_22). Funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflicts of interest
The authors declare that they have no competing interests. Giovanni Benelli is an Editorial Board Member of Parasitology Research. This does not alter the authors’ adherence to all the Parasitology Research policies on sharing data and materials.
Rights and permissions
About this article
Cite this article
Murugan, K., Wei, J., Alsalhi, M.S. et al. Magnetic nanoparticles are highly toxic to chloroquine-resistant Plasmodium falciparum, dengue virus (DEN-2), and their mosquito vectors. Parasitol Res 116, 495–502 (2017). https://doi.org/10.1007/s00436-016-5310-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5310-0