Abstract
Serine protease inhibitors, known as serpins, are pleiotropic regulators of endogenous and exogenous proteases, and molecule transporters. They have been documented in animals, plants, fungi, bacteria, and viruses; here, we characterize a serpin from the trematode platyhelminth Schistosoma mansoni. At least eight serpins have been found in the genome of S. mansoni, but only two have characterized molecular properties and functions. Here, the function of S. mansoni serpin isoform 3 (SmSPI) was analyzed, using both computational and molecular biological approaches. Phylogenetic analysis showed that SmSPI was closely related to Schistosoma haematobium serpin and Schistosoma japonicum serpin B10. Structure determined in silico confirmed that SmSPI belonged to the serpin superfamily, containing nine α-helices, three β-sheets, and a reactive central loop. SmSPI was highly expressed in schistosomules, predominantly in the head gland, and in adult male and female with intensive accumulation on the spines, which suggests that it may have a role in facilitating intradermal and intravenous survival. Recombinant SmSPI was overexpressed in Escherichia coli; the recombinant protein was of the same size (46 kDa) as the native protein. Immunological analysis suggested that mice infected with S. mansoni responded to rSmSPI at 8 weeks postinfection (wpi) but not earlier. The inhibitory activity of rSmSPI was specific to chymotrypsin but not trypsin, neutrophil elastase, and porcine pancreatic elastase. Elucidating the biological and physiological functions of SmSPI as well as other serpins will lead to further understanding of host-parasite interaction machinery that may provide novel strategies to prevent and control schistosomiasis in the future.
Similar content being viewed by others
Introduction
Serine protease inhibitors (serpins) are an important protein superfamily that have key physiological and cell-biological roles in a variety of organisms. All serpins are 330–500 amino acids long, with a molecular weight of ca. 40–60 kDa, and have a conserved structure of three β-sheets, eight or nine α-helices, and a single reactive central loop (RCL) (An et al. 2011; Law et al. 2006). They function as serine protease inhibitors when specific residues (P1-P1′) in the RCL bind with the active site of a protease to form a covalent suicide complex (Khan et al. 2011). The taxa where serpins have been investigated include viruses (Irving et al. 2000), bacteria (Irving et al. 2002), fungi (Steenbakkers et al. 2008), and plants (Roberts and Hejgaard 2008), as well as animals (Gettins 2002), such as members of the genus Schistosoma, which are blood-borne trematode parasites in the Platyhelminthes.
In Schistosoma species, serpins control the homeostasis of serine proteases both in the parasites themselves and their mammalian hosts (Mebius et al. 2013). Serpin in Schistosoma japonicum (SjB10) inhibits human pancreatic elastase in a dose-dependent manner (Molehin et al. 2014b). Parasite-host interaction has also been reported in Schistosoma haematobium (ShSPI) (Blanton et al. 1994). In Schistosoma mansoni, serpin (SmSrpQ) regulates endogenous cercarial elastase but not host serine proteases (Quezada et al. 2012). A S. mansoni serpin (Smpi56) is located on the tegument of adult males and females (Ghendler et al. 1994), which may indicate its role in inhibiting host proteolytic enzymes.
S. mansoni is a major cause of intestinal schistosomiasis, with high morbidity and mortality in several countries worldwide (Gryseels et al. 2006; Hotez et al. 2010; Ittiprasert and Knight 2012). Knowledge of serpins in S. mansoni is relatively limited, despite their importance as targets for immunodiagnosis and vaccines. For example, serpins from S. japonicum are strongly recognized by IgG in sera from rats 6 weeks postinfection (wpi) with a primary challenge (Molehin et al. 2014a, b). Mice vaccinated with S. japonicum serpin had a 36 % decrease in worm burden and a 39 % decrease in (schistosome) egg fecundity (Yan et al. 2005). However, of the eight serpins found in S. mansoni (Granzin et al. 2012), only two have been characterized: Smpi56 (Ghendler et al. 1994) and SmSrpQ (Quezada et al. 2012). Other S. mansoni serpins should also be investigated.
In this study, the full-length S. mansoni serpin isoform 3 (SmSPI) sequence was obtained from NCBI GenBank and then cloned into a prokaryotic expression vector. Computational approaches were used to predict its basic properties and in silico structure, in order to understand its function. The recombinant protein was heterologously expressed in Escherichia coli, and its immunological features and inhibitory functions against target serine proteases were examined.
Materials and methods
Parasites
All developmental stages of S. mansoni were provided by the Applied Malacology Laboratory, Department of Social and Environmental Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Maintenance of the parasite’s life cycle was conducted with the approval of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, in accordance with the Faculty of Tropical Medicine Animal Care and Use Committee guidelines (FTM-ACUC002/2014). Adult parasites were harvested from mice at 60 days postinfection (dpi) according to standard procedures (Duvall and DeWitt 1967; Tucker et al. 2001). Miracidia were induced by light, collected in a conical tube, placed on ice for 30 min, and centrifuged at 6000g and 4 °C for 20 min. Sporocysts were dissected from the hepatopancreas of the intermediate host, Biomphalaria glabrata, at 14 dpi (Ingram et al. 2012; McKerrow et al. 1985). Cercariae were shed from B. glabrata by light stimulation and schistosomules were prepared by in vitro transformation using the two-syringe technique (Milligan and Jolly 2011). Schistosomules and their tails were separated by Percoll gradient centrifugation and then cultured in RPMI-1640 at 37 °C with 5 % CO2 (Lewis 2001) for 2 h. All developmental stages were stored at −70 °C before further use.
Sequence and bioinformatic analysis
The nucleotide and predicted amino acid sequences (accession number CCD60071) of SmSPI were obtained from NCBI GenBank. The amino acid sequence was used for bioinformatic analysis of protein properties, predicting signal peptides using pepstats (Rice et al. 2000) and SignalP 4.1 Server (Petersen et al. 2011), transmembrane helices using TMHMM (Krogh et al. 2001), and potential N- and O-glycosylation sites and disulfide bridges using NetNGlyc 1.0 (Gupta et al. 2004), NetOGlyc 4.0 (Steentoft et al. 2013), and DIpro 2.0 (Román Graván and Cabero Almenara 2013). Canonical signatures of serpins were analyzed using multiple alignment with well-characterized orthologs using Clustal Omega (Sievers et al. 2011). The phylogenetic tree was created by maximum likelihood analysis (ML) with 100 bootstrap replications, using the program MEGA version 5 (Tamura et al. 2011). To examine the degree of sequence conservation at the amino acid level in the RCL, homologous sequences were compared using Sequence LOGO program (Crooks et al. 2004). Accession numbers of all sequences used here are provided in Table 1.
In silico prediction of secondary structure of SmSPI
The secondary structure of SmSPI protein was predicted using the PSIPRED program (Buchan et al. 2013). A three-dimensional structure of SmSPI was generated using Swiss-PdbViewer V.4.04 (Guex and Peitsch 1997) using the S. haematobium serine protease inhibitor (ShSPI) (PDB ID: 3STO) as template. The optimality of the predicted structure was examined using Ramachandran plot analysis implemented on the RAMPAGE server (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php).
RNA isolation and amplification of SmSPI complementary DNA
Total RNA was isolated from miracidium, sporocyst, cercaria, schistosomule, and adult stages of S. mansoni, using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Total RNA from adult S. mansoni (5 μg) was used as the template for first strand complementary DNA (cDNA) synthesis, using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA). SmSPI cDNA was PCR amplified in a reaction volume of 50 μl, containing 2 μl cDNA, 1× Taq polymerase buffer, 0.2 mM of each dNTP, 2 mM MgCl2, 1 U Taq polymerase, and 100 nM SmSPI forward (Fwd) and reverse (Rev) primers. The Fwd primer sequence is 5′-CCGGATCCATGTGTATAAGATTTTCATCAAAAG-3′, where the BamHI restriction site is underlined; the Rev sequence is 3′-AACTGCAGTTATGATGATATAATTGGTTCAATAA-5′, where the PstI restriction site is underlined. PCR conditions were 94 °C for 5 min, followed by 30 cycles of 94 °C for 1 min, 54 °C for 1 min, 72 °C for 1 min, and a final step of 72 °C for 5 min. PCR products were examined using 2 % agarose gel electrophoresis.
Expression of recombinant SmSPI in a prokaryotic expression system
SmSPI cDNA and the pQE30 vector (Qiagen GmbH, Hilden, Germany) were digested with BamHI (Thermo Fisher Scientific) and PstI (Thermo Fisher Scientific) at 37 °C for 2 h and then separated using electrophoresis on a 2 % agarose gel. Bands containing digested DNA fragments were purified using Gel/PCR DNA Fragments Extraction Kit according to the manufacturer’s instructions (Geneaid Biotech Ltd., New Taipei City, Taiwan) and then ligated together using T4 DNA ligase (Thermo Fisher Scientific). The ligation reaction was incubated at 4 °C for 16–18 h, and the pQE30-SmSPI construct was transformed into E. coli JM109 strain using the heat-shock technique (Froger and Hall 2007). Correct insertion of SmSPI into pQE30 was confirmed by DNA sequencing (AIT Biotech, Singapore). To express recombinant SmSPI (rSmSPI), E. coli M15 strain was transformed with pQE30-SmSPI, and then protein expression was induced by incubating with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG; Thermo Fisher Scientific) for 4 h. Bacteria were harvested by centrifugation at 6000g at 4 °C for 30 min, and the pellet was used in the purification of rSmSPI.
Purification of recombinant SmSPI and production of polyclonal antibody
The bacterial pellet was incubated with lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, and 6 M guanidine HCl; pH 8.0) at room temperature for 1 h in a rotary shaker. Lysate was obtained by centrifugation at 14,000g for 30 min and then incubated with Talon® Metal Affinity Resin (Clontech Laboratories, Inc., Mountain View, CA) at 25 °C for 1 h with agitation. After loading the mixture into the Talon® 2 ml Disposable Gravity Column (Clontech Laboratories, Inc.), flow through was collected and then unbound proteins were washed with wash buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole and 8 M urea; pH 6.3). rSmSPI was repeatedly eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, and 8 M urea; pH 6.3) containing an imidazole gradient at 20, 100, and 250 mM. All fractions were analyzed on a 12 % SDS-PAGE gel and stained with Coomassie brilliant blue G-250 (USB Corporation, Cleveland, OH); they were blotted onto a PVDF membrane (Pall Corporation, Ann Arbor, MI) to confirm expression using an anti-His tag antibody (BioLegend®, San Diego, CA). The band containing rSmSPI was excised from the acrylamide gel, digested with trypsin (Sigma-Aldrich Co., St. Louis, MO), and analyzed using a MicroToF Q II mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany). Bioinformatic analysis was as previously described (Adisakwattana et al. 2015; Molee et al. 2015).
Fractions containing rSmSPI were pooled and refolded by gradient dialysis. In detail, rSmSPI was dialyzed in 1× PBS containing 4 M urea for 1 h at RT, and then 1× PBS was gradually added using a peristalsis pump (ATTO Corporation, Tokyo, Japan) with a flow rate of 1 ml/min, until the concentration of urea reached 1 M. Protein concentration was determined using a Coomassie plus (Bradford) assay kit (Thermo Fisher Scientific). A mouse polyclonal antibody, anti-rSmSPI, was produced by GL Biochem Ltd. (Shanghai, China).
Analysis of SmSPI gene expression in different developmental stages
DNA-free total RNA (1 μg) of different developmental stages (miracidium, sporocyst, cercaria, schistosomule, adult male and female) was converted to first strand cDNA as described above. To determine the level of gene expression in each developmental stage, a SYBR green real-time PCR of SmSPI was set up, in 20 μl reactions, containing 2 μl of first strand cDNA, 1× iTaq Universal SYBR® Green (Bio-Rad Laboratories, Inc., Philadelphia, PA), and 100 nM each of Fwd and Rev primers. Specific primers for SmSPI qPCR were Fwd: 5′-AAAGCATTTACTCGGGCGTTTCT-3′ and Rev: 5′-ACCTTGAGCACCACCAGAACCTA-3′. Cytochrome oxidase (COX) was used as an internal control, as described previously (Quezada and McKerrow 2011). Amplification was performed using the LightCycler® 480 II Real-Time PCR System (Roche Applied Science, Mannheim, Germany) with preincubation at 95 °C for 5 min, followed by 45 cycles of 95 °C for 20 s, 54 °C for 20 s, and 72 °C for 30 s. Melting curve analysis was performed at 65–95 °C. The level of gene expression was calculated using the 2−∆∆Ct formula (Livak and Schmittgen 2001), and arbitrary units (A.U.) of expression were subsequently compared among different stages. Experiments were performed with three replicates.
Preparation of parasite antigens
Crude worm antigen (CWA) was prepared by homogenizing adult S. mansoni in RIPA buffer (50 mM Tris-HCl, pH 7.4, 1 % NP-40, 0.25 % sodium deoxycholate, 150 mM NaCl, 1 mM EDTA) containing 1× protease inhibitors (Amresco LLC, Solon, OH) using a glass tissue grinder. To completely break up the cells, five rounds of sonication (Heat System, Newton, CT) with an amplitude of 20 % and 9-s on/off pulse was applied, and the supernatant was collected after centrifugation at 12,000g and 4 °C for 20 min. Excretory-secretory product (ES) was prepared by incubating 50 pairs of adult worms with 10 ml RPMI-1640 (Thermo Fisher Scientific) containing 1× penicillin-streptomycin (Biowest SAS, Nuaillé, France) and 0.45 % glucose, at 37 °C with 5 % CO2 for 16–18 h. The culture medium was collected, dialyzed with 1× PBS for 48 h at 4 °C, and concentrated with Amicon® Stirred Cells (Merck Millipore, Darmstadt, Germany). Protein concentration was measured in both CWA and ES using the Coomassie PlusTM Protein Assay (Thermo Fisher Scientific) according to the manufacturer’s instructions. Proteins were aliquoted into 0.5-ml microfuge tube before storage at −80 °C.
Immunological detection
CWA, ES, and rSmSPI were each electrophoresed using a 12 % SDS-PAGE gel and then electrotransferred onto a PVDF membrane (Pall Corporation). The membrane was blocked with blocking solution (5 % skimmed milk in PBST, i.e., 1× PBS containing 0.05 % Tween 20) at RT for 1 h and then washed thoroughly with PBST. After washing, the membrane was incubated at RT for 1 h, with either a 1:200 dilution of the mouse polyclonal antibody against rSmSPI, or mouse infected sera at 0, 2, 4, 6, and 8 wpi in blocking solution and then with HRP-conjugated anti-mouse IgG at RT for 1 h. For detection of native SmSPI in CWA and ES, the membrane was incubated with a SuperSignal® West Dura Extended Duration Substrate (Thermo Fisher Scientific) and then imaged using the ImageQuant LAS 4000 mini (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). For detection of S. mansoni-infected mouse sera against rSmSPI, the membrane was incubated with 2,6-dichloroindophenol (Sigma-Aldrich) for color development, as previously described (Nuamtanong et al. 2012).
Immunolocalization of SmSPI in the parasite tissue
Cercariae or schistosomules were permeabilized by incubation with 1× PBS containing 0.1 % Triton X-100 for 10 min. After that, the solution containing the parasite was dropped onto poly-l-lysine-coated slides (Electron Microscopy Sciences, Hatfield, PA) and allowed to air dry at ambient temperature. Slides were fixed with acetone for 10 min in a humid chamber and subsequently incubated with mouse anti-rSmSPI serum (1:100) in the humid chamber at 4 °C for 16–18 h. After washing three times with 1× PBS, a 1:500 dilution of FITC-anti-mouse IgG (BioLegend) was added and incubated for 1 h at RT in the dark in the humid chamber. Slides were then washed with 1× PBS three times and incubated with 1:10,000 DAPI for 5 min, followed by five washes with 1× PBS. Samples were mounted with 50 % glycerol, covered with a coverslip, and sealed with nail polish. Slides were kept in the dark and immediately examined using a LSM700 confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany).
Fresh adult male and female were immediately fixed in 4 % paraformaldehyde in PBS, pH 7.4, and then paraffin-embedded prior to cut at 5 μm thick. An immunohistochemistry was performed as described previously with slight modification (Adisakwattana et al. 2007). Briefly, paraffin sections were initially processed including dewaxing, rehydrating, retrieving antigenic epitopes, and neutralizing endogenous peroxidase, respectively. After that, nonspecific binding was blocked with 5 % (v/v) fetal bovine serum (FBS) in 1× PBS, pH 7.4, followed by incubating with mouse anti-rSmSPI (1:100) or preimmune sera (1:100), and subsequently horse radish peroxidase (HRP)-conjugated goat anti-mouse Ig (1:1000; SouthernBiotech, Birmingham, AL). The chromogenic result was developed using AEC staining kit (Sigma-Aldrich) according to the manufacturer’s instructions.
Determination of the inhibitory property of recombinant SmSPI
Trypsin (Sigma-Aldrich), chymotrypsin (Sigma-Aldrich), neutrophil elastase (Fitzgerald Industries International, Acton, MA), and porcine pancreatic elastase (Calbiochem® EMD Chemicals, Inc., San Diego, CA) were used as target serine proteases to determine the inhibitory activity of rSmSPI. Assays were performed as per previous publications but with some modification (Del Mar et al. 1980; Gaertner and Puigserver 1992; Nakajima et al. 1979; Sandanaraj et al. 2005). In summary, each serine protease was mixed with rSmSPI at molar ratios of (enzyme/inhibitor) 2:0, 1:1, 1:2, 1:4, and 0:2 and then incubated at 37 °C for 30 min. The colorimetric substrate of trypsin (Nα-benzoyl-l-arginine 4-nitroanilide hydrochloride; Sigma-Aldrich), chymotrypsin (N-succinyl-Gly-Gly-Phe-p-nitroanilide; Sigma-Aldrich), porcine pancreatic elastase (elastase substrate IV; Calbiochem®), or neutrophil elastase (elastase substrate I; Calbiochem®) was added to a final concentration of 0.1, 0.75, 0.9, and 0.9 mM, respectively. Optical density (OD) at 405 nm was kinetically measured using a SunriseTM microplate reader (Tecan Group Ltd., Männedorf, Switzerland). PMSF was used as a control, at a concentration of 20 μM. Stoichiometry of inhibition (SI) was determined by incubating chymotrypsin (0.2 μM) with various concentrations of rSmSPI (0, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, and 2 μM) and then interacted with chymotrypsin substrate (0.75 mM) as described previously (Toubarro et al. 2013). The inhibition constant (K i) of the chymotrypsin-rSmSPI complex was performed as described elsewhere with slight modification (Toubarro et al. 2013). In summary, a constant concentration of chymotrypsin (2 μM) was incubated with different concentrations of rSmSPI (0, 0.5, 1, and 2 μM) for 10 min at 37 °C, and then varying concentrations of chymotrypsin substrate (0.75, 1, and 2 μM) were added into the reactions. The estimated K i value was determined using a Dixon plot.
Results
Bioinformatic analysis of the SmSPI sequence
The coding sequence of SmSPI comprises a 1218-bp open reading frame (ORF) encoding a protein of 406 amino acid residues. Molecular weight and isoelectric point (pI) calculated using pepstats (EMBOSS) suggested that the protein was 46 kDa with a pI of 5.95. Signal peptide and transmembrane helix prediction analysis showed that SmSPI did not contain either signal peptides or a transmembrane region. Potential glycosylation sites were found in the sequence at residues N16, N97, N152, N249, N325, and N349 for N-glycosylation and at S75, S78, and S79 for O-glycosylation (Fig. 1). Cysteine residue and disulfide bond analysis (using DIpro 2.0) showed that SmSPI contains three cysteine residues at C2, C42, and C383 but no disulfide bonds. Sequence similarity to protein orthologs was examined using BLASTP and suggested that SmSPI was similar to proteins in the serpin superfamily, particularly those of Platyhelminthes of closely related Schistosoma spp. The top hits were ShSPI, followed by SjB10 (see Table 2). Multiple alignment was performed for the identification of conserved sequence motifs, comparing SmSPI with other serpins, including ShSPI, SjB10, and those from Clonorchis sinensis (CsSerp), Paragonimus westermani (PwSerp), and Echinococcus multilocularis (EmSerp). The amino acid sequence of SmSPI was highly homologous to ShSPI and SjB10 (Fig. 2). The putative RCL consensus residues of SmSPI were identified based on conserved serpin RCL peptides “P17 [E]-P16 [E/K/R]-P15 [G]-P14 [T/S]-P13 [X]-P12–9 [AGS]-P8–1 [X]-P1′–4′” (Mulenga et al. 2009). The RCL of SmSPI was present at amino acid residues 350–370 (ESGIEATTVTSPIFVPISAVL), containing Ile and Ser at P1 and P1′, respectively. A serpin signature sequence was found at the C-terminus of SmSPI at amino acid positions 375–385 (FNVNHPFICFI) (Fig. 2). A phylogenetic tree was constructed using 17 orthologs derived from nematode, trematode, cestode, human, the amoebozoan Entamoeba histolytica, and the apicomplexan Eimeria tenella. SmSPI is closely related to ShSPI and SjB10 but distinctively separated from other serpins of human, amoebozoan, apicomplexan, S. mansoni, and other trematodes or cestodes (Fig. 3). Nematode serpins were located the farthest away from SmSPI in the tree.
Multiple alignment of serpin amino acid sequences indicates conserved regions of the serpin superfamily. Identical and similar residues are indicated by black and gray shading, respectively. Gaps are indicated by a dash (-). The RCL is indicated by a bold arrow above the region and serpin signature is indicated by a dashed arrow above the region. P1 and P1′ residues are indicated by asterisk and number sign, respectively. Accession numbers of all amino acid sequences used in this analysis are presented in Table 1
To identify the conserved sequence motifs of the RCL in Schistosoma spp., amino acid sequences at P17-P4′ of Schistosoma serpin were analyzed using WebLogo. The consensus amino acid of Schistosoma RCL is “P17 [E]-P16 [X]-P15 [G]-P14 [I/A/V]-P13 [E/V]-P12–9 [ATV]-P8–1 [X]-P1′–2′ [SA]-P3′–4′” with P1 containing either nonpolar—isoleucine (I), phenylalanine (F), or leucine (L)—or polar amino acid—arginine (R) and P1′ containing serine (S) (Fig. 4a, b).
Structural modeling of SmSPI
Prediction of SmSPI secondary structure showed that this protein is composed of nine α-helices and 14 β-sheets (Fig. 5a). The tertiary structure was determined by homology modeling. Firstly, a template structure was identified, with ShSPI having the greatest homology to SmSPI. SmSPI had a similar structure to that of ShSPI, which contained nine α-helices, three β-sheets, and one RCL (Fig. 5b). Ramachandran plotting suggested that the structural model was optimal (data not shown).
Predicted secondary structure (a) was determined using the 3STO tertiary structure as a template; “H” indicates helices, “B” indicates sheets, and the box indicates the RCL. The predicted tertiary structure of SmSPI (b), based on ShSPI, using 3STO, had nine α-helices, three β-sheets, and the RCL (arrow)
Cloning and expression of recombinant SmSPI
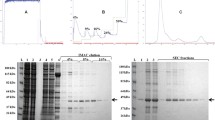
cDNA of SmSPI was amplified from adult S. mansoni RNA with a molecular size of 1218 bp, and the DNA sequence was identical to that in GenBank (CCD60071) (Fig. 6a). Bacterially expressed rSmSPI was produced as insoluble protein with a molecular size of 46 kDa (Fig. 6b) and was detected by the anti-His tag antibody (Fig. 6c). rSmSPI was purified under denaturing conditions and then refolded by stepwise dialysis, showing a predominant protein molecular size of 46 kDa (Fig. 6d). Mass spectrometry results confirmed that the expressed recombinant protein is SmSPI (data not shown).
Cloning, expression, and purification of rSmSPI. a SmSPI cDNA was amplified and determined to be 1212 bp, shown here compared with the 100-bp plus DNA marker, Thermo Fisher Scientific (M). b cDNA was subcloned into the pQE30 expression vector and then expressed in M15 E. coli using 1 mM IPTG; M broad range prestained protein marker, NI noninducing, ID inducing. c Western blot analysis with mouse anti-His tag antibodies confirmed rSmSPI expression at approximately 46 kDa; M broad range prestained protein marker, NI noninducing, ID inducing. d SDS-PAGE gel showing purified rSmSPI after Co2+ affinity chromatography; M broad range prestained protein marker (Thermo Fisher Scientific), 1 purified rSmSPI
Determination of SmSPI transcript in different developmental stages of S. mansoni
SmSPI gene was expressed in all developmental stages: miracidium, sporocyst, cercaria, schistosomule, and adult male and female (Fig. 7). The highest level of expression was found in schistosomules (0.225 A.U.), which was approximately 2–20 times higher than in other stages. Surprisingly, cercariae (0.0006 A.U.) had the lowest levels, 20 times less than schistosomules. Adult males (0.012 A.U.) had slightly higher levels than adult females (0.0076 A.U.). Miracidia (0.0025 A.U.) and sporocysts (0.004 A.U.) had low levels of SmSPI transcripts, but these were higher than in cercariae.
Transcriptional analysis of SmSPI expression in different developmental stages of S. mansoni using SYBR real-time RT-PCR. Mi miracidium, Spor sporocyst, Cer cercaria, Schis schistosomule, AM adult male, AF adult female. The expression level is measured in arbitrary units as described in the “Materials and methods” (A.U.)
Immunological detection of SmSPI
Western blotting of rSmSPI with mouse anti-rSmSPI antisera showed a strong reaction at the expected size of 46 kDa, but also weakly detected truncated forms at 7–20 kDa (Fig. 8a). In crude parasite extracts, mouse anti-rSmSPI antibody could detect native SmSPI in adult parasites, at 46 kDa, similar to rSmSPI (Fig. 8a). However, the antisera could not detect SmSPI in ES products of adult parasites, using both colorimetric and chemiluminescent substrates (data not shown). Western blotting of rSmSPI with S. mansoni-infected mouse sera at 2, 4, 6, and 8 wpi showed negative results for 2, 4, and 6 wpi (data not shown), while at 8 wpi, there was a strong reaction with intact rSmSPI (46 kDa) and truncated proteins (Fig. 8b). Probing with preimmune sera was negative in all experiments.
Western blot analysis detecting native SmSPI in a crude extract of S. mansoni adult worm (a) showed the protein reacted at the molecular size of 46 kDa, the same as rSmSPI; 1: CWA, 2: rSmSPI. The immune response against rSmSPI was determined by western blot (b), which showed that 8 wpi mouse sera can detect rSmSPI, M mouse, −: noninfected sera, +: S. mansoni-8 wpi sera
In situ localization of SmSPI in cercariae, schistosomules, and adult S. mansoni
In previous publications, SmSrpQ was found to be highly expressed in cercariae and predominantly localized to the pre- and postacetabulum (Davies 1983; Dorsey and Stirewalt 1971; Fishelson et al. 1992). However, our study found that SmSPI was upregulated in schistosomules but less so in cercariae. Immunolocalization of SmSPI was performed in both cercariae and schistosomules. In cercariae, SmSPI antibody staining showed predominant expression in the head region with weak expression at the tail (Fig. 9; upper). In schistosomules, SmSPI was localized over the whole parasite with a majority of expression at the head gland (Fig. 9; lower). In adult male and female, SmSPI was localized in parenchyma and tegument with predominant concentration on the spines (Fig. 10). Preimmunized sera were used as a negative control.
Immunolocalization of SmSPI in tissue of adult female (a–c) and male (d–f) S. mansoni. a Section of adult female. b High magnification of adult female section. c Control of adult female reacted with mouse preimmune sera. d Section of adult male. e High magnification of adult male section. f Control of adult male reacted with mouse preimmune sera. Arrow indicates spines of adult female and male S. mansoni
Determination of inhibitory activity of recombinant SmSPI
Inhibition by serpins typically occurs when serine proteases bind to serpins and form irreversible covalent complexes. In this study, inhibitory assays of SmSPI to trypsin, chymotrypsin, neutrophil elastase, and porcine pancreatic elastase were performed using a colorimetric substrate. After incubating SmSPI with target proteases for 30 min, SmSPI was found to partially inhibit chymotrypsin. Increasing the molar ratio of rSmSPI suggested that the activity of chymotrypsin was decreased (Fig. 11a). Other serine proteases (trypsin, neutrophil elastase, and porcine pancreatic elastase) could not be inhibited by rSmSPI (data not shown). An inhibitory effect of rSmSPI over chymotrypsin was demonstrated as a weak inhibition (SI = 25.35) (Fig. 11b) and low affinity binding (K i = 1.77 μM) (Fig. 11c).
Inhibitory activity of rSmSPI against chymotrypsin. a Increasing concentrations of rSmSPI in the reaction markedly impair chymotrypsin activity. Different molar ratios of chymotrypsin/rSmSPI was used at 2:0, 1:1, 1:2, 1:4, and 0:2. PMSF was used as a control serine protease inhibitor. b Stoichiometry of inhibition (SI) of rSmSPI determined against chymotrypsin. c Inhibition constant (K i) of rSmSPI was 1.77 μM for complex with chymotrypsin
Discussion
Evasion and modulation of host immunity by parasitic helminths are indispensable mechanisms that facilitate the parasite’s survival, growth, and development. Recently, several strategies used by trematode parasites have been demonstrated, including inhibition of host proteases through their specific inhibitors (Lee and Fidock 2008; McKerrow et al. 1999; O’Brien et al. 2008; Pina-Vazquez et al. 2012). Protease inhibitors are groups of proteins that play important roles in the regulation of homeostasis in both parasites and their hosts (Quezada and McKerrow 2011). Several classes of protease inhibitor have been identified and characterized, such as cysteine-, aspartic-, metallo-, and serine protease inhibitors (Knox 2007). Serine protease inhibitors or serpins are essential proteins that helminths use for regulating host immunity by inhibition of serine protease-associated immune functions, e.g., apoptosis (Heutinck et al. 2010), coagulation (Loof et al. 2011), granzyme killing (Niehaus et al. 2015; Pieter et al. 2011), complement activation (Takahashi et al. 2007), and neutrophil functions (Pham 2006). In this study, the serpin of S. mansoni isoform 3, namely SmSPI, was molecularly characterized using bioinformatics, molecular biology, and immunology to elucidate its basic functions and properties.
Sequence analysis of SmSPI showed that this protein is composed of 406 amino acids with an expected molecular size of 46 kDa, without signal peptides or transmembrane helices. These suggest that SmSPI is an intracellular protein that may have a crucial role in protease homeostasis, as well as being a molecular chaperone. However, the excretory-secretory potential needs to be investigated further, as several proteins (including serpins) without signal peptides can be found in parasite excretory-secretory products, mediated by a nonclassical secretory mechanism (Moreno and Geary 2008; Ragg 2007). Multiple N- and O-glycosylation sites predicted in SmSPI indicate that posttranslational modification may potentially occur in native SmSPI. In ShSPI and SjB10, glycosylation sites have been observed in native forms that increase the molecular size of the protein in crude worm extract, compared to recombinant proteins (Blanton et al. 1994; Molehin et al. 2014b). Three cysteine residues were present in the SmSPI amino acid sequence, but these did not predict disulfide bridge formation. These results may indicate that the tertiary structure of SmSPI does not depend upon cysteine interaction but would rely on unique interactions between amino acid composition in α-helices and β-sheets (Gettins 2002).
Multiple sequence alignment suggested that SmSPI has high homology with ShSPI and SjB10, with the RCL sequence being particularly conserved. The RCL of SmSPI was identified on the basis of the inhibitory serpin consensus sequence “P17 [E]-P16 [E/K/R]-P15 [G]-P14 [T/S]-P13 [X]-P12–9 [AGS]-P8–1 [X]-P1′–P4′” (Mulenga et al. 2009). However, the RCL of SmSPI and its closely related neighbors, ShSPI and SjB10, showed some variations: P16 [S], P14 [I], and P12–9 [ATV]. A specific consensus sequence for Schistosoma would be (on the basis of current sampling): “P17 [E]-P16 [X]-P15 [G]-P14 [I/A/V]-P13 [E/V]-P12–9 [ATV]-P8–1 [X]-P1′–2′ [SA]-P3′–4′.” This may indicate different activity or specificity of serpins in Schistosoma as opposed to in other taxa. The hinge region (P15–9) plays an important role by turning the RCL to join with a β-sheet, as strand 5A, which is necessary for the inhibitory properties of serpins. Nonconservation of amino acids at the hinge region results in impairment of normal function or a complete loss of inhibitory activity (Hopkins et al. 1993; Zhang et al. 2015). Therefore, the mutation in the hinge region found in schistosomes may affect inhibitory properties, and this requires further clarification. The phylogenetic analysis of SmSPI with other orthologs strongly confirmed its homology and close relatedness to ShSPI and SjB10. All this suggests that the well-characterized ShSPI (Granzin et al. 2012) and SjB10 (Molehin et al. 2014b) can be used to guide studies of the basic structure, properties, and function of SmSPI. However, the SmSPI, ShSPI, and SjB10 clade was separated away from other parasite and human serpins, which may indicate some unique characteristic and/or function of this group. This should be considered as a further topic for exploration.
Secondary and tertiary structures of SmSPI were constructed in silico using ShSPI as a template. SmSPI contains nine α-helices and 14 β-sheets, which is typical of classical serpins (Molehin et al. 2012). The predicted tertiary structure of SmSPI was nine α-helices, three incorporated β-sheets, and a single RCL, as also present in ShSPI. However, an unusual configuration of the helical subdomains (hC-hD) found in the ShSPI tertiary structure (Granzin et al. 2012) should be explored in SmSPI by protein crystallography.
Recombinant SmSPI was heterologously expressed in E. coli with a molecular size of 46 kDa, which is the same size as the native protein. This indicates that no posttranslational modification through glycosylation occurs in native SmSPI. Conversely, native ShSPI and SjB10 each shows a high degree of glycosylation. Molecular characterization of another S. mansoni serpin, namely SmSrpQ, found that glycosylation could not be detected in the native protein even in the presence of several glycosylation sites (Quezada et al. 2012). This implies that the glycosylation machinery of S. mansoni serpins may differ from that of other schistosomes, though other potentially remaining isoforms should also be investigated. Gene expression analysis of SmSPI in different developmental stages demonstrated that SmSPI was highly expressed in schistosomules and adult males but not cercariae, as reported previously for SmSrpQ (Quezada et al. 2012). These data suggest that upregulation of different isoforms may be associated with stage-specific serine proteases expressed in the parasite. Trypsin- and chymotrypsin-like serine proteases of S. mansoni are differentially expressed throughout its developmental stages; serine protease isoform 2 (SP2) is highly expressed in schistosomules and adults (Horn et al. 2014). These may be related to the expression of SmSPI in schistosomules and adult males, but this requires further clarification. In addition to parasite serine proteases, SmSPI expression may be regulated by tissue-specific serine proteases of its host. Several serine proteases produced by human skin act as innate defensive weapons against pathogens, including schistosomes (Lim et al. 1999); blockage of these skin serine proteases might facilitate the survival of schistosomules. Localization of SmSPI in schistosomules suggests that expression of SmSPI is distributed over the whole body with the highest expression at the tip of the head. This head tip location may support SmSPI’s potential role in host-parasite interaction, inhibiting a target serine protease in the host skin. Localization of SmSrpQ was predominantly observed in cercariae in the pre- and postacetabular glands and head glands. In schistosomules, SmSrpQ was localized to pre- and postacetabular glands (Quezada et al. 2012). These observations support the hypothesis above that upregulation of each serpin isoform may be dependent on specific types of both parasite and host tissue serine proteases. In adult S. mansoni, SmSPI was predominantly accumulated at the body surface of both male and female, which is consistent with the location of ShSPI in S. haematobium (Blanton et al. 1994). Although SmSPI could not be detected in ES of adult parasite, the surface exposure of this protein may support its role in host-parasite interaction. Another evidence toward host-parasite interaction was suggested by reacting SmSPI with 8 wpi mouse sera which gave a positive result that is consistent with the previously observed immunoreactivity of infected rat sera against SjB10 (Molehin et al. 2014a). The presence of SmSPI in the extracellular environment supports other observations of a rise in antibody titer in S. mansoni-infected human sera, compared to healthy controls (Tanigawa et al. 2015). These strongly suggest that SmSPI has a role in host-parasite interaction that should be further investigated, with a view to the development of immunodiagnosis and vaccines.
The inhibitory activity of SmSPI was restricted to chymotrypsin but was not observed with other serine proteases (trypsin, pancreatic elastase, and neutrophil elastase). The inhibitory effect of SmSPI on chymotrypsin demonstrated a weak inhibition (SI = 25.35) with low affinity binding (K i = 1.77 M), which may be due to inappropriate protein folding produced by E. coli (Jayakumar et al. 2004, “Materials and methods”). There were different substrate preferences between SmSPI and SjB10, even through their P1-P1′ (Ile-Ser) are the same. SjB10 inhibited porcine pancreatic elastase with dose dependence but not chymotrypsin as in SmSPI (Molehin et al. 2014b). However, the variability in the hinge region at P11–10 and P4 found in both serpins may have an impact on their inhibitory activity and substrate specificity. An Ala → Thr mutation at P10 of a C1 inhibitor stopped covalent complex formation with C1s (Davis et al. 2007). Variation at P4, from hydrophobic Ile to amino acids with different properties—polar uncharged (Gly, Ser), positively charged (Arg), negatively charged (Glu), or bulk hydrophobic (Phe, Trp)—has an impact on the activity of anti-thrombin by decreasing the rate of complex formation (Cunningham et al. 1997).
In conclusion, the third isoform of S. mansoni serpin (SmSPI) was successfully cloned and heterologously expressed as a recombinant protein in E. coli. Regions of conservation and secondary and tertiary structures were predicted in silico. Immunological properties and protein functions were also demonstrated in this study, supporting the potential of SmSPI as a target in immunodiagnosis and vaccine development. Further studies of SmSPI should include in vivo inhibition of this protein using interference RNA or gene knockouts; other isoforms in the genome of S. mansoni should also be investigated to understand the complete role of serpins in this important schistosome parasite.
References
Adisakwattana P, Viyanant V, Chaicumpa W, Vichasri-Grams S, Hofmann A, Korge G, Sobhon P, Grams R (2007) Comparative molecular analysis of two asparaginyl endopeptidases and encoding genes from Fasciola gigantica. Mol Biochem Parasitol 156(2):102–116. doi:10.1016/j.molbiopara.2007.07.006
Adisakwattana P, Suwandittakul N, Petmitr S, Wongkham S, Sangvanich P, Reamtong O (2015) ALCAM is a novel cytoplasmic membrane protein in TNF-α stimulated invasive cholangiocarcinoma cells. Asian Pac J Cancer Prev 16(9):3849–3856
An C, Ragan EJ, Kanost MR (2011) Serpin-1 splicing isoform J inhibits the proSpätzle-activating proteinase HP8 to regulate expression of antimicrobial hemolymph proteins in Manduca sexta. Dev Comp Immunol 35(1):135–141. doi:10.1016/j.dci.2010.09.004
Blanton RE, Licate LS, Aman RA (1994) Characterization of a native and recombinant Schistosoma haematobium serine protease inhibitor gene product. Mol Biochem Parasitol 63(1):1–11
Buchan DWA, Minneci F, Nugent TCO, Bryson K, Jones DT (2013) Scalable web services for the PSIPRED protein analysis workbench. Nucleic Acids Res. doi:10.1093/nar/gkt381
Crooks GE, Hon G, Chandonia J-M, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14(6):1188–1190. doi:10.1101/gr.849004
Cunningham MA, Blajchman MA, Sheffield WP (1997) Impact of mutations at the P4 and P5 positions on the reaction of antithrombin with thrombin and elastase. Thromb Res 88(2):171–181
Davies TW (1983) Schistosoma mansoni: the structure and elemental composition of pre-acetabular penetration gland cell secretion in pre-emergent cercariae. Parasitology 87(Pt 1):55–60
Davis AE 3rd, Cai S, Liu D (2007) C1 inhibitor: biologic activities that are independent of protease inhibition. Immunobiology 212(4–5):313–323
Del Mar EG, Largman C, Brodrick JW, Fassett M, Geokas MC (1980) Substrate specificity of human pancreatic elastase 2. Biochemistry (Mosc) 19(3):468–472
Dorsey CH, Stirewalt MA (1971) Schistosoma mansoni: fine structure of cercarial acetabular glands. Exp Parasitol 30(2):199–214. doi:10.1016/0014-4894(71)90084-1
Duvall RH, DeWitt WB (1967) An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg 16(4):483–486
Fishelson Z, Amiri P, Friend DS, Marikovsky M, Petitt M, Newport G, McKerrow JH (1992) Schistosoma mansoni: cell-specific expression and secretion of a serine protease during development of cercariae. Exp Parasitol 75(1):87–98. doi:10.1016/0014-4894(92)90124-S
Froger A, Hall JE (2007) Transformation of plasmid DNA into E. coli using the heat shock method. J Vis Exp (6):253 doi:10.3791/253
Gaertner HF, Puigserver AJ (1992) Increased activity and stability of poly(ethylene glycol)-modified trypsin. Enzym Microb Technol 14(2):150–155
Gettins PGW (2002) Serpin structure, mechanism, and function. Chem Rev 102(12):4751–4804. doi:10.1021/cr010170+
Ghendler Y, Arnon R, Fishelson Z (1994) Schistosoma mansoni: isolation and characterization of Smpi56, a novel serine protease inhibitor. Exp Parasitol 78(2):121–131. doi:10.1006/expr.1994.1013
Granzin J, Huang Y, Topbas C, Huang W, Wu Z, Misra S, Hazen SL, Blanton RE, Lee X, Weiergraber OH (2012) Three-dimensional structure of a schistosome serpin revealing an unusual configuration of the helical subdomain. Acta Crystallogr D Biol Crystallogr 68(Pt 6):686–694. doi:10.1107/S0907444912008372
Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368(9541):1106–1118. doi:10.1016/S0140-6736(06)69440-3
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723. doi:10.1002/elps.1150181505
Gupta R, Jung E, Brunak S (2004) NetNGlyc 1.0 Server. http://www.cbs.dtu.dk/services/NetNGlyc/
Heutinck KM, ten Berge IJM, Hack CE, Hamann J, Rowshani AT (2010) Serine proteases of the human immune system in health and disease. Mol Immunol 47(11–12):1943–1955. doi:10.1016/j.molimm.2010.04.020
Hopkins PC, Carrell RW, Stone SR (1993) Effects of mutations in the hinge region of serpins. Biochemistry (Mosc) 32(30):7650–7657
Horn M, Fajtová P, Rojo Arreola L, Ulrychová L, Bartošová-Sojková P, Franta Z, Protasio AV, Opavský D, Vondrášek J, McKerrow JH, Mareš M, Caffrey CR, Dvořák J (2014) Trypsin- and chymotrypsin-like serine proteases in Schistosoma mansoni—‘the undiscovered country’. PLoS Negl Trop Dis 8(3):e2766. doi:10.1371/journal.pntd.0002766
Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A (2010) Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol 8(11):814–826
Ingram JR, Rafi SB, Eroy-Reveles AA, Ray M, Lambeth L, Hsieh I, Ruelas D, Lim KC, Sakanari J, Craik CS, Jacobson MP, McKerrow JH (2012) Investigation of the proteolytic functions of an expanded cercarial elastase gene family in Schistosoma mansoni. PLoS Negl Trop Dis 6(4):e1589. doi:10.1371/journal.pntd.0001589
Irving JA, Pike RN, Lesk AM, Whisstock JC (2000) Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res 10(12):1845–1864. doi:10.1101/gr.147800
Irving JA, Steenbakkers PJM, Lesk AM, Op den Camp HJM, Pike RN, Whisstock JC (2002) Serpins in prokaryotes. Mol Biol Evol 19(11):1881–1890
Ittiprasert W, Knight M (2012) Reversing the resistance phenotype of the Biomphalaria glabrata snail host Schistosoma mansoni infection by temperature modulation. PLoS Pathog 8(4):e1002677. doi:10.1371/journal.ppat.1002677
Jayakumar A, Cataltepe S, Kang Y, Frederick MJ, Mitsudo K, Henderson Y, Crawford SE, Silverman GA, Clayman GL (2004) Production of serpins using baculovirus expression systems. Methods 32(2):177–184. doi:10.1016/S1046-2023(03)00209-3
Khan MS, Singh P, Azhar A, Naseem A, Rashid Q, Kabir MA, Jairajpuri MA (2011) Serpin inhibition mechanism: a delicate balance between native metastable state and polymerization. J Amino Acids 2011:606797. doi:10.4061/2011/606797
Knox DP (2007) Proteinase inhibitors and helminth parasite infection. Parasite Immunol 29(2):57–71. doi:10.1111/j.1365-3024.2006.00913.x
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305(3):567–580. doi:10.1006/jmbi.2000.4315
Law RHP, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI, Whisstock JC (2006) An overview of the serpin superfamily. Genome Biol 7(5):216. doi:10.1186/gb-2006-7-5-216
Lee MCS, Fidock DA (2008) Arresting malaria parasite egress from infected red blood cells. Nat Chem Biol 4(3):161–162
Lewis F (2001) Schistosomiasis. Current protocols in immunology. Wiley, New York
Lim KC, Sun E, Bahgat M, Bucks D, Guy R, Hinz RS, Cullander C, McKerrow JH (1999) Blockage of skin invasion by schistosome cercariae by serine protease inhibitors. Am J Trop Med Hyg 60(3):487–492
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Loof TG, Mörgelin M, Johansson L, Oehmcke S, Olin AI, Dickneite G, Norrby-Teglund A, Theopold U, Herwald H (2011) Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood 118(9):2589–2598. doi:10.1182/blood-2011-02-337568
McKerrow JH, Pino-Heiss S, Lindquist R, Werb Z (1985) Purification and characterization of an elastinolytic proteinase secreted by cercariae of Schistosoma mansoni. J Biol Chem 260(6):3703–3707
McKerrow JH, Engel JC, Caffrey CR (1999) Cysteine protease inhibitors as chemotherapy for parasitic infections. Bioorg Med Chem 7(4):639–644. doi:10.1016/S0968-0896(99)00008-5
Mebius MM, van Genderen PJJ, Urbanus RT, Tielens AGM, de Groot PG, van Hellemond JJ (2013) Interference with the host haemostatic system by schistosomes. PLoS Pathog 9(12):e1003781. doi:10.1371/journal.ppat.1003781
Milligan JN, Jolly ER (2011) Cercarial transformation and in vitro cultivation of Schistosoma mansoni schistosomules. J Vis Exp 54:3191. doi:10.3791/3191
Molee P, Adisakwattana P, Reamtong O, Petmitr S, Sricharunrat T, Suwandittakul N, Chaisri U (2015) Up-regulation of AKAP13 and MAGT1 on cytoplasmic membrane in progressive hepatocellular carcinoma: a novel target for prognosis. Int J Clin Exp Pathol 8(9):9796–9811
Molehin AJ, Gobert GN, McManus DP (2012) Serine protease inhibitors of parasitic helminths. Parasitology 139(6):681–695. doi:10.1017/S0031182011002435
Molehin AJ, Gobert GN, Driguez P, McManus DP (2014a) Characterisation of a secretory serine protease inhibitor (SjB6) from Schistosoma japonicum. Parasit Vectors 7:330. doi:10.1186/1756-3305-7-330
Molehin AJ, Gobert GN, Driguez P, McManus DP (2014b) Functional characterization of SjB10, an intracellular serpin from Schistosoma japonicum. Parasitology 141(13):1746–1760. doi:10.1017/S0031182014001061
Moreno Y, Geary TG (2008) Stage- and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS Negl Trop Dis 2(10):e326. doi:10.1371/journal.pntd.0000326
Mulenga A, Khumthong R, Chalaire KC (2009) Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics 10:217. doi:10.1186/1471-2164-10-217
Nakajima K, Powers JC, Ashe BM, Zimmerman M (1979) Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem 254(10):4027–4032
Niehaus JZ, Miedel MT, Good M, Wyatt AN, Pak SC, Silverman GA, Luke CJ (2015) SERPINB12 is a slow-binding inhibitor of granzyme A and hepsin. Biochemistry (Mosc) 54(45):6756–6759. doi:10.1021/acs.biochem.5b01042
Nuamtanong S, Dekumyoy P, Adisakwattana P (2012) Evaluation of recombinant serine protease inhibitor from Trichinella spiralis for immunodiagnosis of swine trichinosis. Southeast Asian J Trop Med Public Health 43(5):1094–1104
O’Brien TC, Mackey ZB, Fetter RD, Choe Y, O’Donoghue AJ, Zhou M, Craik CS, Caffrey CR, McKerrow JH (2008) A parasite cysteine protease is key to host protein degradation and iron acquisition. J Biol Chem 283(43):28934–28943. doi:10.1074/jbc.M805824200
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8(10):785–786, http://www.nature.com/nmeth/journal/v8/n10/abs/nmeth.1701.html#supplementary-information
Pham CTN (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6(7):541–550
Pieter JADK, Kummer JA, de Poot SAH, Quadir R, Broekhuizen R, McGettrick AF, Higgins WJ, Devreese B, Worrall DM, Bovenschen N (2011) Intracellular serine protease inhibitor SERPINB4 inhibits granzyme M-induced cell death. PLoS One 6(8):e22645. doi:10.1371/journal.pone.0022645
Pina-Vazquez C, Reyes-Lopez M, Ortiz-Estrada G, de la Garza M, Serrano-Luna J (2012) Host-parasite interaction: parasite-derived and -induced proteases that degrade human extracellular matrix. J Parasitol Res 2012:748206. doi:10.1155/2012/748206
Quezada LA, McKerrow JH (2011) Schistosome serine protease inhibitors: parasite defense or homeostasis? An Acad Bras Cienc 83(2):663–672
Quezada LA, Sajid M, Lim KC, McKerrow JH (2012) A blood fluke serine protease inhibitor regulates an endogenous larval elastase. J Biol Chem 287(10):7074–7083. doi:10.1074/jbc.M111.313304
Ragg H (2007) The role of serpins in the surveillance of the secretory pathway. Cell Mol Life Sci 64(21):2763–2770. doi:10.1007/s00018-007-7157-0
Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16(6):276–277. doi:10.1016/S0168-9525(00)02024-2
Roberts T, Hejgaard J (2008) Serpins in plants and green algae. Funct Integr Genomics 8(1):1–27. doi:10.1007/s10142-007-0059-2
Román Graván P, Cabero Almenara J (2013) Analítica web de la comunidad virtual DIPRO2.0 / web analytics of virtual community DIPRO2.0. RELATEC 12(1):35–50
Sandanaraj BS, Vutukuri DR, Simard JM, Klaikherd A, Hong R, Rotello VM, Thayumanavan S (2005) Non-covalent modification of chymotrypsin surface using amphiphilic polymeric scaffold—implications in modulating protein function. J Am Chem Soc 127(30):10693–10698. doi:10.1021/ja051947+
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi:10.1038/msb.2011.75
Steenbakkers PJM, Irving JA, Harhangi HR, Swinkels WJC, Akhmanova A, Dijkerman R, Jetten MSM, van der Drift C, Whisstock JC, Op den Camp HJM (2008) A serpin in the cellulosome of the anaerobic fungus Piromyces sp. strain E2. Mycol Res 112(8):999–1006. doi:10.1016/j.mycres.2008.01.021
Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KTBG, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Paul Bennett E, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H (2013) Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J 32(10):1478–1488. doi:10.1038/emboj.2013.79
Takahashi M, Mori S, Shigeta S, Fujita T (2007) Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. Adv Exp Med Biol 598:93–104
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739. doi:10.1093/molbev/msr121
Tanigawa C, Fujii Y, Miura M, Nzou SM, Mwangi AW, Nagi S, Hamano S, Njenga SM, Mbanefo EC, Hirayama K, Mwau M, Kaneko S (2015) Species-specific serological detection for schistosomiasis by serine protease inhibitor (SERPIN) in multiplex assay. PLoS Negl Trop Dis 9(8):e0004021. doi:10.1371/journal.pntd.0004021
Toubarro D, Avila MM, Montiel R, Simões N (2013) A pathogenic nematode targets recognition proteins to avoid insect defenses. PLoS One 8(9):e75691. doi:10.1371/journal.pone.0075691
Tucker MS, Karunaratne LB, Lewis FA, Freitas TC, Liang Y-s (2001) Schistosomiasis. Current protocols in immunology. Wiley, New York
Yan Y, Liu S, Song G, Xu Y, Dissous C (2005) Characterization of a novel vaccine candidate and serine proteinase inhibitor from Schistosoma japonicum (Sj serpin). Vet Parasitol 131(1–2):53–60. doi:10.1016/j.vetpar.2005.04.038
Zhang X, Meekins DA, An C, Zolkiewski M, Battaile KP, Kanost MR, Lovell S, Michel K (2015) Structural and inhibitory effects of hinge loop mutagenesis in serpin-2 from the malaria vector Anopheles gambiae. J Biol Chem 290(5):2946–2956. doi:10.1074/jbc.M114.625665
Acknowledgments
This study was supported by grant FY2013 from the Faculty of Tropical Medicine, Mahidol University. We thank Suthep Numnuan and Songtham Kiatsiri from the Applied Malacology Laboratory, Department of Social and Environmental Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, for providing S. mansoni throughout this study. Our gratitude also goes to the Faculty of Tropical Medicine for funding the proofreading, editing, and page charging of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Pattarakul Pakchotanon and Patamaporn Molee contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pakchotanon, P., Molee, P., Nuamtanong, S. et al. Molecular characterization of serine protease inhibitor isoform 3, SmSPI, from Schistosoma mansoni . Parasitol Res 115, 2981–2994 (2016). https://doi.org/10.1007/s00436-016-5053-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5053-y