Abstract
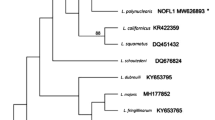
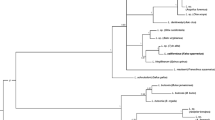
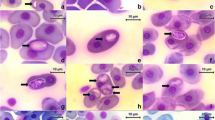
We describe morphologically unique Leucocytozoon pterotenuis sp. nov. (Haemosporida, Leucocytozoidae), the first reported leucocytozoid species developing in fusiform host cell found in a Neotropical passeriform bird. The type host of this parasite is the Chestnut-crowned Antpitta (Grallaria ruficapilla, Grallariidae), an elusive native passerine bird whose natural history remains, to a large degree, unexplored. This bird was captured in Palacio forest in the damping zone of Chingaza National Natural Park, Cundinamarca, Colombia, at 2900 m above sea level (asl). Gametocytes of the new species develop both in roundish and fusiform host cells. This parasite is readily morphologically distinguishable from the described Leucocytozoon species because its host cells possess the narrow (needle-like) spindle-shaped processes, which length markedly exceeds their width. Additionally, the host cell nucleus markedly extends into the processes. Phylogenetic relationships were constructed based on a fragment of the mitochondrial cytochrome b gene and the complete mitochondrial genome. Phylogenetic analysis placed the lineage of L. pterotenuis in different positions depending on the length of the sequence analyzed that is likely due to poor sampling of Leucocytozoon species, especially from rare or non-passerine hosts, as well as a paucity of complete mitochondrial sequences of these parasites. Available data indicate that Leucocytozoon parasites are distributed mainly in mountain regions of the Neotropics where unique morphological forms have been recently discovered. To a better knowledge of the diversity of Leucocytozoon spp. and their host–vector–parasite interactions in Neotropical countries, additional deep and intensive samplings are needed, particularly in orders different to Passeriformes.



Similar content being viewed by others
References
Belo NO, Pinheiro RT, Reis ES, Ricklefs RE, Braga ÉM (2011) Prevalence and lineage diversity of avian haemosporidians from three distinct cerrado habitats in Brazil. PLoS One 6:e17654. doi:10.1371/journal.pone.0017654
Bennett GF, Witt H, White EM (1980) Blood parasites of some Jamaican birds. J Wildl Dis 16:29–38. doi:10.7589/0090-3558-16.1.29
Bennett GF, Montgomerie R, Seutin G (1992) Scarcity of haematozoa in birds breeding on the Arctic Tundra of North America. Condor 94:289–292. doi:10.2307/1368821
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hansson B, Westerdahl H et al (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc Biol Sci 267:1583–1589. doi:10.1098/rspb.2000.1181
Bensch S, Pérez-Tris J, Waldenström J, Hellgren O (2004) Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution 58:1617–1621. doi:10.1111/j.0014-3820.2004.tb01742.x
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
BirdLife I (2014) Species factsheet: Grallaria ruficapilla. In: Chestnut-crowned Antpitta Grallaria ruficapilla. http://www.birdlife.org/datazone/species/factsheet/22703274. Accessed 10 Apr 2014
Cadena D, Estacio H, Lucero R, Erazo N (2012) Plan de desarrollo “Es tiempo de avanzar”. http://www.pupiales-narino.gov.co/apc-aa-files/33663063366461626161306634386632/plan-de-desarrollo-pupiales-2012-2015.pdf. Accessed 13 Oct 2014
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Meth 9:772–772. doi:10.1038/nmeth.2109
Dimitrov D, Zehtindjiev P, Bensch S, Ilieva M, Iezhova T, Valkiūnas G (2014) Two new species of Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) from European birds, with emphasis on DNA barcoding for detection of haemosporidians in wildlife. Syst Parasitol 87:135–151. doi:10.1007/s11230-013-9464-1
Escalante AA, Freeland DE, Collins WE, Lal AA (1998) The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc Natl Acad Sci U S A 95:8124–8129. doi:10.1073/pnas.95.14.8124
Forrester DJ, Greiner EC (2008) Leucocytozoonosis. In: Atkinson CT, Thomas NJ, Hunter (eds) Parasitic diseases of wild birds. Blackwell Publishing, Ames, Iowa, pp 54–107
Forrester DJ, Foster GW, Morrison JL (2001) Leucocytozoon toddi and Haemoproteus tinnunculi (Protozoa: Haemosporina) in the Chimango caracara (Milvago chimango) in southern Chile. Mem Inst Oswaldo Cruz. doi:10.1590/S0074-02762001000700024
Funk DJ, Omland KE (2003) Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu Rev Ecol Evol Syst 34:397–423. doi:10.1146/annurev.ecolsys.34.011802.132421
Gabaldon A, Ulloa G (1976) Encuesta sobre malaria aviaria en Venezuela: resultados del tercer y último año. Bol Mal Salud Amb 16:107–117
Gabaldon A, Ulloa G, Montcourt A (1974) Encuesta sobre malaria aviaria en Venezuela: resultados del primer año. Bol Mal Salud Amb 14:80–103
Gabaldon A, Ulloa G, Montcourt A (1975) Encuesta sobre malaria aviaria en Venezuela. Resultados del segundo año. Bol Mal Salud Amb 15:73–91
Galen SC, Witt CC (2014) Diverse avian malaria and other haemosporidian parasites in Andean house wrens: evidence for regional co-diversification by host-switching. J Avian Biol 45:374–386. doi:10.1111/jav.00375
Galindo P, Sousa O (1966) Blood parasites of birds from Almirante, Panama with ecological notes on the hosts. Rev Biol Trop 14:27–46
Gálvez CF, Ramírez GF, Osorio JH (2010) Parámetros hematológicos de la mirla Mimus gilvus (paseriformes: mimidae) en cautiverio. Bol Cient Mus Hist Nat Univ Caldas 14:120–128
Gosler A (2004) Birds in the hand. Bird ecology and conservation: a handbook of techniques. Oxford University Press, p 91
Greiner EC (1976) Leucocytozoon maccluri sp. n. (Haemosporida: Leucocytozoidae) from a Thailand Thrush, Zoothera marginata Blyth. J Parasitol 62:545–547. doi:10.2307/3279409
Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi:10.1093/sysbio/syq010
Hauptmanova K, Literak I, Bartova E (2002) Haematology and leucocytozoonosis of Great Tits (Parus major L.) during winter. Acta Vet Brno 71:199–204. doi:10.2754/avb200271020199
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802. doi:10.1645/GE-184R1
Herzog SK, Kattan GA (2011) Patrones de diversidad y endemismo en las aves de los Andes tropicales. In: Herzog SK, Martinez R, Jorgensen PM, Tiessen H (eds) Cambio climatico y biodiversidad en los Andes tropicales. Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), Paris, pp 287–305
IUCN (2014) The IUCN Red List of Threatened Species. Version 2014.2. http://www.iucnredlist.org/. Accessed 30 Oct 2014
Josse C, Cuesta F, Navarro G, Barrena V, Cabrera E, Chacón-Moreno E et al (2009) Mapa de Ecosistemas de los Andes del Norte y Centro. Bolivia, Colombia, Ecuador, Perú y Venezuela. Secretaría General de la Comunidad Andina, Programa Regional ECOBONA, CONDESAN-Proyecto Paramo Andino, Programa BioAndes, EcoCiencia, NatureServe, LTA-UNALM, IAvH, ICAE-ULA, CDCUNALM, RUMBOL SRL, Lima
Kattan GH, Beltran JW (1999) Altitudinal distribution, habitat use, and abundance of Grallaria antpittas in the Central Andes of Colombia. Bird Conserv Int 9:271–281. doi:10.1017/S0959270900003452
Krabbe NK, Schulenberg TS (2004) Family Formicariidae (ground antbirds). In: Hoyo J, Elliott A, Christie D (eds) Handbook of the birds of the world (broadbills to tapaculos). Lynx Edicions, Barcelona, pp 682–731
Lacorte GA, Félix GMF, Pinheiro RRB, Chaves AV, Almeida-Neto G, Neves FS et al (2013) Exploring the diversity and distribution of Neotropical avian malaria parasites a molecular survey from Southeast Brazil. PLoS One 8:e57770. doi:10.1371/journal.pone.0057770
Levin II, Valkiūnas G, Iezhova TA, O’Brien SL, Parker PG (2012) Novel Haemoproteus species (Haemosporida: Haemoproteidae) from the swallow-tailed gull (Lariidae), with remarks on the host range of hippoboscid-transmitted avian hemoproteids. J Parasitol 98:847–854. doi:10.1645/GE-3007.1
Levin II, Zwiers P, Deem SL, Geest EA, Higashiguchi JM, Iezhova TA et al (2013) Multiple lineages of avian malaria parasites (Plasmodium) in the Galapagos Islands and evidence for arrival via migratory birds: Plasmodium in galapagos birds. Conserv Biol 27:1366–1377. doi:10.1111/cobi.12127
Lotta IA, Matta NE, Torres RD, Sandino MM, Moncada LI (2013) Leucocytozoon fringillinarum and Leucocytozoon dubreuili in Turdus fuscater from a Colombian páramo ecosystem. J Parasitol 99:359–362. doi:10.1645/GE-3156.1
Mantilla JS, González AD, Valkiūnas G, Moncada LI, Matta NE (2013a) Description and molecular characterization of Plasmodium (Novyella) unalis sp. nov. from the Great Thrush (Turdus fuscater) in highland of Colombia. Parasitol Res 112:4193–4204. doi:10.1007/s00436-013-3611-0
Mantilla JS, Matta NE, Pacheco MA, Escalante AA, González AD, Moncada LI (2013b) Identification of Plasmodium (Haemamoeba) lutzi (Lucena, 1939) from Turdus fuscater (Great Thrush) in Colombia. J Parasitol 99:662–668. doi:10.1645/12-138.1
Marzal A, García-Longoria L, Cárdenas Callirgos JM, Sehgal RN (2014) Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol Invasions. doi:10.1007/s10530-014-0718-x
Matta NE, Lotta IA, Valkiūnas G, González AD, Pacheco MA, Escalante AA et al (2014a) Description of Leucocytozoon quynzae sp. nov. (Haemosporida, Leucocytozoidae) from hummingbirds, with remarks on distribution and possible vectors of leucocytozoids in South America. Parasitol Res 113:457–468. doi:10.1007/s00436-013-3675-x
Matta NE, Pacheco MA, Escalante AA, Valkiūnas G, Ayerbe-Quiñones F, Acevedo-Cendales LD (2014b) Description and molecular characterization of Haemoproteus macrovacuolatus n. sp. (Haemosporida, Haemoproteidae), a morphologically unique blood parasite of black-bellied whistling duck (Dendrocygna autumnalis) from South America. Parasitol Res 113:2991–3000. doi:10.1007/s00436-014-3961-2
McMullan M, Quevedo A, Donegan G (2011) Guia de Campo de las Aves de Colombia. Fundación ProAves, Bogotá
Miller M., Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE), 2010. IEEE, New Orleans, LA, pp 1–8
Moritz C, Cicero C (2004) DNA barcoding: promise and pitfalls. PLoS Biol 2:e354. doi:10.1371/journal.pbio.0020354
Muñoz E, Ferrer D, Molina R, Adlard RD (1999) Prevalence of haematozoa in birds of prey in Catalonia, north-east Spain. Vet Rec 144:632–636. doi:10.1136/vr.144.23.632
Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R (2011) Avian malaria deaths in parrots, Europe. Emerg Infect Dis 17:950–952. doi:10.3201/eid1705.101618
Pacheco MA, Battistuzzi FU, Junge RE, Cornejo OE, Williams CV, Landau I, Rabetafika L, Snounou G, Jones-Engel L, Escalante AA (2011a) Timing the origin of human malarias: the lemur puzzle. BMC Evol Biol 11:299. doi:10.1186/1471-2148-11-299
Pacheco MA, Escalante AA, Garner MM, Bradley GA, Aguilar RF (2011b) Haemosporidian infection in captive masked bobwhite quail (Colinus virginianus ridgwayi), an endangered subspecies of the northern bobwhite quail. Vet Parasitol 182:113–120. doi:10.1016/j.vetpar.2011.06.006
Pacheco M, Cranfield M, Cameron K, Escalante AA (2013) Malarial parasite diversity in chimpanzees: the value of comparative approaches to ascertain the evolution of Plasmodium falciparum antigens. Malar J 12:328. doi:10.1186/1475-2875-12-328
Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S (2011) Plasmodium relictum (lineage SGS1) and Plasmodium ashfordi (lineage GRW2): the effects of the co-infection on experimentally infected passerine birds. Exp Parasitol 127:527–533. doi:10.1016/j.exppara.2010.10.007
Peirce MA (1984) Haematozoa of Zambian birds. IV. Description of Leucocytozoon balmorali sp. nov. from Malaconotidae. J Nat Hist 18:223–226. doi:10.1080/00222938400770181
Pérez Ponce de León G, García L (2001) Los parásitos en el contexto de la biodivrsidad y la conservación. CONABIO. Biodiversitas 34:11–15
Pérez-Tris J, Bensch S (2005) Diagnosing genetically diverse avian malarial infections using mixed-sequence analysis and TA-cloning. Parasitology 131:15–23
Perkins SL, Martinsen ES, Falk BG (2011) Do molecules matter more than morphology? Promises and pitfalls in parasites. Parasitology 138:1664–1674. doi:10.1017/S0031182011000679
Poulin R, Krasnov BR, Mouillot D, Thieltges DW (2011) The comparative ecology and biogeography of parasites. Phil Trans R Soc B 366:2379–2390. doi:10.1098/rstb.2011.0048
Rambaut A (2006) FigTree Tree Figure Drawing Toll version 1.3.1. Institute of Evolutionary Biology, University of Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 31 Jan 2013
Remsen JV, Cadena CD, Jaramillo A, Nores M, Pacheco JF, Pérez-Emán J et al (2012) A classification of the bird species of South America American Ornithologists’ Union Version 7 December 2012. http://www.museum.lsu.edu/~Remsen/SACCBaseline.html. Accessed 7 Dec 2012
Rodríguez OA, Moya H, Matta NE (2009) Avian blood parasites in the National Natural Park Chingaza: high Andes of Colombia. Hornero 24:1–6
Rokas A, Carroll SB (2005) More genes or more taxa? The relative contribution of gene number and taxon number to phylogenetic accuracy. Mol Biol Evol 22:1337–1344. doi:10.1093/molbev/msi121
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual, Second. Cold Spring Harbor Laboratory Press, New York
Santiago-Alarcon D, Palinauskas V, Schaefer HM (2012) Diptera vectors of avian Haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol Rev Camb Philos Soc 87:928–964. doi:10.1111/j.1469-185X.2012.00234.x
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Meth 9:671–675. doi:10.1038/nmeth.2089
Soltis DE, Albert VA, Savolainen V, Hilu K, Qiu Y-L, Chase MW et al (2004) Genome-scale data, angiosperm relationships, and “ending incongruence”: a cautionary tale in phylogenetics. Trends Plant Sci 9:477–483. doi:10.1016/j.tplants.2004.08.008
Svensson-Coelho M, Blake JG, Loiselle BA, Penrose AS, Parker PG, Ricklefs RE (2013) Diversity, prevalence, and host specificity of avian Plasmodium and Haemoproteus in a western Amazon assemblage. Ornithol Monogr 76:1–47. doi:10.1525/om.2013.76.1.1
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Toft CA, Karter AJ (1990) Parasite-host coevolution. Trends Ecol Evol 5:326–329. doi:10.1016/0169-5347(90)90179-H
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiunas G, Iezhova TA, Mironov SV (2002) Leucocytozoon hamiltoni n. sp. (Haemosporida, Leucocytozoidae) from the Bukharan Great Tit Parus bokharensis. J Parasitol 88:577. doi:10.2307/3285453
Valkiūnas G, Salaman P, Iezhova TA (2003) Paucity of hematozoa in Colombian birds. J Wildl Dis 39:445–448. doi:10.7589/0090-3558-39.2.445
Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Sehgal RNM, Bensch S (2008) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401. doi:10.1645/GE-1570.1
Valkiūnas G, Sehgal RNM, Iezhova TA, Hull AC (2010) Identification of Leucocytozoon toddi group (Haemosporida: Leucocytozoidae), with remarks on the species taxonomy of leucocytozoids. J Parasitol 96:170–177. doi:10.1645/GE-2109.1
Valkiūnas G, Palinauskas V, Ilgūnas M, Bukauskaitė D, Dimitrov D, Bernotienė R et al (2014) Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitol Res 113:2251–2263. doi:10.1007/s00436-014-3880-2
Vargas-Rios O, Pedraza P, Universidad Nacional de Colombia (Bogota), Departamento de Biologia (2004) El Parque Nacional Natural Chingaza. Gente Nueva, Bogotá, D.C.
Vásquez VH, Serrano MA (2009) Las áreas naturales protegidas de Colombia. Conservación Internacional-Colombia & Fundación Biocolombia., Bogotá, D.C.
White EM, Greiner EC, Bennett GF, Herman CM (1978) Distribution of the hematozoa of neotropical birds. Rev Biol Trop 26(Suppl 1):43–102
Acknowledgments
Authors would like to thank Chingaza National Natural Park Administrative staff and Grupo de Estudio Relación Parásito Hospedero research group, especially Rocio Hernandez and Rafael Gutierrez, for their field assistance. This work was funded by Colciencias project code 110152128340 contract no. 359–2011 and the Botanical Garden José Celestino Mutis of Bogotá under program of incentives for research Thomas van der Hammen.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lotta, I.A., Gonzalez, A.D., Pacheco, M.A. et al. Leucocytozoon pterotenuis sp. nov. (Haemosporida, Leucocytozoidae): description of the morphologically unique species from the Grallariidae birds, with remarks on the distribution of Leucocytozoon parasites in the Neotropics. Parasitol Res 114, 1031–1044 (2015). https://doi.org/10.1007/s00436-014-4269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4269-y