Abstract
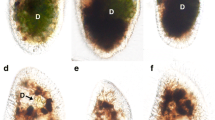
The study deals with the pleomorphic zooflagellate Histomonas meleagridis, which was cultivated under different stress conditions to induce a possible encystation. In the present paper, the morphological changes were analyzed by light and electron microscopy. The determination of the proliferation under different adverse conditions led to conclusions on the tenacity of the flagellate. H. meleagridis parasitizes in the intestinal tract of galliform birds and may cause enormous losses in poultry farming. For the development of new therapy approaches, clarification of the transmission pathways will be helpful. Different clonal cultures of H. meleagridis established by micromanipulation and exposed to media lacking different ingredients, inappropriate temperatures, and/or distinct reagents were investigated. Lowering of temperature was proven to have adverse effects on the survival of H. meleagridis. The flagellate could not survive in a frozen medium, and survival in a temperature of 4°C lasted no longer than 23 h. An addition of sodium chloride induced an increased proliferation; pH values between 2 and 8 set limits for the survival of the parasite in different ways. H. meleagridis was able to survive under high acidic conditions for only 1 h. The major amount of cells, which could be discovered in the controls, measured 8–12 μm appeared amoebic (stage 1) and were filled with enclosures of rice starch. A rounding of most cells was noted 4 h at 4°C after incubation in minimal essential medium in the absence of rice starch and fetal calf serum. A higher osmolarity of the medium, which was initiated by the addition of sodium chloride or magnesium chloride, did not induce an encystation process. After addition of hypochlorite base and cultivating at pH values between 7 and 8, spherical stages without a flagellum were formed (stage 2) measuring about 8–12 μm in diameter. Their interior consisted of a central and a peripheral region when studied by transmission electron microscopy. This aspect was due to the location of the glycogen granules. The central zone was described as totally filled with the carbohydrates, which made totally invisible the other organelles. The solidity of the amorphous layer below the cell membrane seemed to hinder the invasion of the glycogen granules. The amorphous layer below the cell membrane made it apparently possible that the cell might survive under adverse conditions—at least for a short time. This special structure might enable H. meleagridis to proceed a fast transmission and to infect many birds in a rather short time, which was shown in the past by several studies. Double-membraned cells, which were guessed to be cyst-like structures of the parasite, were also detected (stage 3). The size of these cells, however, was much smaller than that of the amoebic stages or the above-described spherical forms of H. meleagridis. Furthermore, the small cells were characterized by other granula structures. These findings might be interpreted that the small stages are possibly long-term (true) cysts and that the spherical stages with the amorphous layer beneath the cell membrane might be short-term cysts. Both, however, should be able to survive situations outside of a body and thus might be transmitted from feces to another animal.







Similar content being viewed by others
References
Duffy CF, Sims MD, Power RF (2005) Evaluation of dietary Natustat for control of Histomonas meleagridis in male turkeys on infected litter. Avian Dis 49:423–425
Esquenet C, De Herdt P, De Bosschere H, Ronsmans S, Ducatelle R, Van Erum J (2003) An outbreak of histomoniasis in free-range layer hens. Avian Pathol 32:305–308
Grabensteiner E, Hess M (2006) PCR for the identification and differentiation of Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis spp. Vet Parasitol 142:230–233
Graybill HW, Smith T (1920) Production of fatal blackhead in turkeys by feeding embryonated eggs of Heterakis papillosa. J Exp Med 31:647–655
Hess M, Grabensteiner E (2003) Aktuelle Bedeutung der Histomoniasis (Schwarzkopfkrankheit) beim Wirtschaftsgeflügel. Lohmann Information 3/2003:1–3
Hess M, Grabensteiner E, Liebhart D (2006a) Rapid transmission of the protozoan parasite Histomonas meleagridis in turkeys an specific pathogen-free chickens following cloacal infection with monoeucaryotic culture. Avian Pathol 35:280–285
Hess M, Kolbe T, Grabensteiner E, Prosl H (2006b) Clonal cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis sp. established through micromanipulation. Parasitol Res 133:547–554
Hu J, McDougald LR (2003) Direct lateral transmission of Histomonas meleagridis in turkeys. Avian Dis 47:489–492
Hu J, Fuller L, McDougald LR (2004) Infection of turkeys with Histomonas meleagridis by the cloacal drop method. Avian Dis 48:746–750
Huber K, Gouilloud L, Zenner L (2007) A preliminary study of natural and experimental infection of the lesser mealworm Alphitobius diaperinus (Coleoptera: Tenebrionidae) with Histomonas meleagridis (Protozoa: Sarcomastigophora). Avian Pathol 36:279–282
Hussein EM, Atwa MM (2008) Infectivity of Trichomonas vaginalis pseudocysts intra-vaginally in mice. J Egypt Soc Parasitol 38:749–762
Levine ND (1973) Protozoan parasites of domestic animals and of man. Burgess, Minneapolis, pp 88–110
Liebhart D, Hess M (2009) Oral infection of turkeys with vitro-cultured Histomonas meleagridis results in high mortality. Avian Pathol 38:223–227
Lund EE (1972) Histomoniasis. Dis Poult 9:990–1006
Lund EE, Chute AM, Wilkins GC (1975) The wild turkey as a host for Heterakis gallinarum and Histomonas meleagridis. J Wildl Dis 11:376–381
McDougald LR (2005) Blackhead disease (histomoniasis) in poultry: a critical review. Avian Dis 49:462–476
Mehlhorn H, Haberkorn A, Schaub G (1981) Studies on Blastocrithidia triatomae. Acta Trop 56:210–218
Mehlhorn H, Hansen O, Mencke N (1999) Effects of imidacloprid on adult or larvae stages of the flea Ctenocephalides felis after in-vivo and in-vitro application. Parasitol Res 85:625–637
Mehlhorn H, Al-Quraishy S, Aziza A, Hess M (2009) Fine structure of the bird parasites Trichomonas gallinae and Tetratrichomonas gallinarum from cultures. Parasitol Res 105:751–756
Mielewczik M, Mehlhorn H, Al-Quraishy S, Grabensteiner E, Hess M (2008) Transmission electron microscopic studies of stages of Histomonas meleagridis from clonal cultures. Parasitol Res 103:745–750
Munsch M, Lotfi A, Hafez M, Al-Quraishy S, Mehlhorn H (2008) Light and transmission electron studies on trophozoites and cyst-like stages of Histomonas meleagridis from cultures. Parasitol Res 104:683–689
Norton PA, Clark FD, Beasley JN (1999) An outbreak of histomoniasis in turkeys infected with a moderate level of Ascaria dissimilis but no Heterakis gallinarum. Avian Dis 43:342–348
Schuster FL (1968) Ultrastructure of Histomonas meleagridis (Smith) Tyzzer, a parasitic amoebo-flagellate. J Parasitol 54:725–737
Tyzzer EE (1919) Development phases of the protozoan of blackhead in turkeys. J Med Res 40:1–30
Tyzzer EE, Collier J (1925) Induced and natural transmission in the absence of Heterakis. J Infect Dis 37:265–276
Zaragatzki E, Mehlhorn H, Abdel-Ghaffar F, Rasheid KAS, Grabensteiner E, Hess M (2010) Experiments to produce cysts in cultures of Histomonas meleagridis the agent of histomonosis in poultry. Parasitol Res (in press)
Acknowledgement
We are grateful to the Center of Excellence of the King Saud University (College of Science), Riyadh, Saudi Arabia for their support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaragatzki, E., Hess, M., Grabensteiner, E. et al. Light and transmission electron microscopic studies on the encystation of Histomonas meleagridis . Parasitol Res 106, 977–983 (2010). https://doi.org/10.1007/s00436-010-1777-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1777-2