Abstract
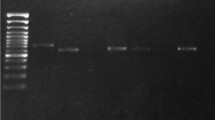
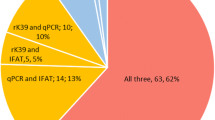
The laboratory diagnosis of visceral leishmaniasis is based on microscopic examination, culture, serological tests, and molecular methods. In this study, we examined 50 blood specimens from suspected visceral leishmaniasis patients by microscopic examination, recombinant antigen dipstick test (rK39), and polymerase chain reaction (PCR) in the University of Cukurova, Faculty of Medicine, Parasitology Department in Turkey. We calculated the sensitivity–specificity and positive–negative predictive values for these diagnostic tests. We found that positive predictive value of microscopy examination, rK39 dipstick test, and PCR were 20%, 24%, and 58% for visceral leishmaniasis, respectively. When we compared polymerase chain reaction, recombinant antigen dipstick test, and microscopic examination for visceral leishmaniasis diagnosis, the polymerase chain reaction is more sensitive (100%) than recombinant antigen dipstick test and microscopy examination.

Similar content being viewed by others
References
Al-Nahhas S, Shabaan M, Hammoud L, Al-Taweel A, Al-Jort S (2003) Visceral leishmaniasis in the Syrian Arab Republic: early detection using rK39. East Mediterr Health J 9(4):856–862
Bensoussan E, Nasereddin A, Jonas F, Schnur LF, Jaffe CL (2006) Comparison of PCR assay for diagnosis of cutaneous leishmaniasis. J Clin Microbiol 44:1435–1439
Carvalho SF, Lemos EM, Corey R, Dietze R (2003) Performance of recombinant K39 antigen in the diagnosis of Brazilian visceral leishmaniasis. Am J Trop Med Hyg 68(3):321–324
Chappuis F, Rijial S, Soto A, Menten J, Boelaert M (2006) A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ 333:723–726
Disch J, Caligiorne RB, Maciel F, Oliveira MC, Orsini M, Dias-Neto E, Rabello A (2006) Single-step duplex kDNA-PCR for detection of Leishmania donovani complex in human peripheral blood samples. Diagn Microbiol Infect Dis 56:395–400
Fissore C, Delaunay P, Ferrua B, Rosenthal E, Del Giudice P, Aufeuvre JP, Le Fichoux Y, Marty P (2004) Convenience of serum for visceral leishmaniasis diagnosis by PCR. 42(11):5332–5333
Gangneux JP, Menotti J, Lorenzo F, Sarfati C, Blanche H, Bui H, Pratlong F, Garin YJ, Derouin F (2003) Prospective value of PCR amplification and sequencing for diagnosis and typing of old world leishmania infections in area of nonendemicity. J Clin Microbiol 41(4):1419–1422
Herwaldt BL (1999) Leishmaniasis. Lancet 354:1191–1199
Iqbal J, Hira PR, Saroj G, Philip R, Al-Ali F, Madda PJ, Sher A (2002) Imported visceral leishmaniasis: diagnostic dilemmas and comparative analysis of three assays. J Clin Microbiol 40(2):475–479
Lachaud L, Marchergui-Hammami S, Chabbert E, Dereure J, Dedet JP, Bastien P (2002) Comparision of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J Clin Microbiol 40(1):210–215
Marfurt J, Niederwieser I, Makia ND, Beck HP, Felger I (2003) Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis 46(2):115–124
Mary C, Faraut F, Lascombe L, Dumon H (2004) Quantification of Leishmania infantum DNA by real-time PCR assay with high sensitivity. J Clin Microbiol 42(11):5249–5255
Motazedian H, Karamian M, Noyes HA, Ardehali S (2002) DNA extraction and amplification of Leishmania from archived, Giemsa-stained slides, for the diagnosis of cutaneous leishmaniasis by PCR. Ann Trop Med Parasitol 96(1):31–34
Nicolas L, Millon G, Prina E (2002) Rapid differentiation of old world Leishmania species by lightcycler polymerase chain reaction and melting curve analysis. J Microbiol Methods 51:295–299
Noyes HA, Reyburn H, Bailey JW, Smith D (1998) A nested PCR based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J Clin Microbiol 36:2877–2881
Osman OF, Oskam L, Zıjlstra EE, Kroon CM, Schoone GJ, Khalil AG, El-Hassan AM, Kager PA (1997) Evaluation of PCR for diagnosis of visceral leishmaniasis. J Clin Microbiol 35(10):2454–2457
Pal S, Aggarwal G, Haldar A, Majumdar A, Majumdar HK, Duttagupta S (2004) Diagnosis of symptomatic kala-azar by polymerase chain reaction using patient’s blood. Med Sci Monit 10(1):MT1–MT5
Piarroux R, Gambarell F, Dumon H, Fontes M, Dunan S, Mary C, Toga B, Quilici M (1994) Comparison of PCR with direct examination of bone marrow aspiration, myeloculture and serology for diagnosis of visceral leishmaniasis in immunocompromised patients. J Clin Microbiol 32(3):746–749
Reithinger R, Dujardin JC (2007) Molecular diagnosis of leishmaniasis: current status and future applications. J Clin Microbiol 45(1):21–25
Salotra P, Sreenivas G, Pogue GP, Lee N, Nakhasi HL, Ramesh V, Negi NS (2001) Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis. J Clin Microbiol 39(3):849–854
Schonian G, Fari M, Lewin S, Schweynoch C, Presber W (2001) Molecular epidemiology and population genetics in Leishmania. Med Microbiol Immunol 190(1–2):61–63
Sciberras R (2007) The use of K39 test in the diagnosis of visceral leishmaniasis. Malta Med J 19:42–45
Sharma NL, Mahajan VK, Negi AK, Verma GK (2009) The rK39 immunochromatic dipstick testing: a study for K39 seroprevalence in dogs and human leishmaniasis patients for possible animal reservoir of cutaneous and visceral leishmaniasis in endemic focus of Satluj river valley of Himachal Pradesh (India). Indian J Dermatol Venerol Leprol 75(1):52–55
Silva ES, Gontijo CMF, Pacheco RS, Brazil RP (2004) Diagnosis of human visceral leishmaniasis by PCR using blood samples spotted on filter paper. Genet Mol Res 3(2):251–257
Sundar S, Rai M (2002) Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol 9(5):951–958
Sundar S, Reed SG, Singh VP, Kumar PC, Murray HW (1998) Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet 351(9102):563–565
Veeken H, Ritmeijer K, Seaman J, Davidson R (2003) Comparison of an rK39 dipstick rapid test with direct agglutination test and splenic aspiration fort the diagnosis of kala-azar in Sudan. Trop Med Int Health 8(2):164–167
Zijlstra EE, Ali MS, El-Hassan AM, El-Toum IA, Satti M, Ghalib HW, Kager PA (1992) Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans R Soc Trop Med Hyg 86(5):505–507
Acknowledgments
This project was supported by the Cukurova University Research Grant TF.2005.D4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozerdem, D., Eroglu, F., Genc, A. et al. Comparison of microscopic examination, rK39, and PCR for visceral leishmaniasis diagnosis in Turkey. Parasitol Res 106, 197–200 (2009). https://doi.org/10.1007/s00436-009-1650-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1650-3