Abstract
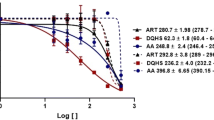
The lysosomal cysteine proteinase activity of bloodstream forms of Trypanosoma brucei is a validated drug target. Previously, it was reported that nitric oxide (NO)-releasing agents inhibit the catalytic activity of cysteine proteinases of the protozoan parasites Leishmania infantum, Trypanosoma cruzi and Plasmodium falciparum. In this study, we investigated the effect of the NO-donors S-nitrosoglutathione, (±)-(E)-4-ethyl-2[(E)-hydroxyimino]-5-nitro-3-hexenamide, 3-morpholinosydnonimine (SIN-1) and S-nitroso-N-acetyl-dl-penicillamine on the activity of the cysteine proteinase of T. brucei. At a concentration of 1 mM, the NO donors inhibited the catalytic activity of purified T. brucei cysteine proteinase by 50–90%. With the exception of SIN-1, all NO donors displayed trypanocidal activities against bloodstream forms of T. brucei in vitro with 50% growth inhibition values of around 30 μM. However, the NO donors were ineffective in significantly inhibiting the cysteine proteinase activity within the parasites. This finding was confirmed by the ineffectiveness of the NO donors to block proteinolysis in the lysosome of the parasites. The results show that the trypanocidal activity of NO donors cannot be attributed to the inhibition of the major lysosomal cysteine proteinase in bloodstream forms of T. brucei.




Similar content being viewed by others
References
Ascenzi P, Bocedi A, Gentile M, Visca P, Gradoni L (2004) Inactivation of parasite cysteine proteinases by the NO-donor 4-(phenylsulfonyl)-3-((2-(dimethylamino)ethyl)thio)-furoxan oxalate. Biochim Biophys Acta 1703:69–77
Baltz T, Baltz D, Giroud C, Crockett J (1985) Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J 4:1273–1277
Barrett AJ, Kirschke H (1981) Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol 80:535–561
Bocedi A, Gradoni L, Menegatti E, Ascenzi P (2004) Kinetics of parasite cysteine proteinase inactivation by NO-donors. Biochem Biophys Res Commun 315:710–718
Bourguignon SC, Alves CR, Giovanni-De-Simone S (1997) Detrimental effect of nitric oxide on Trypanosoma cruzi and Leishmania major like cells. Acta Trop 66:109–118
Caffrey CR, Hansell E, Lucas KD, Brinen LS, Alvarez Hernandez A, Cheng J, Gwaltney SL 2nd, Roush WR, Stierhof YD, Bogyo M, Steverding D, McKerrow JH (2001) Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense. Mol Biochem Parasitol 118:61–73
Clark IA, Rockett KA (1996) Nitric oxide and parasitic disease. Adv Parasitol 37:1–56
Fairlamb AH (2003) Chemotherapy of human African trypanosomiasis: current and future prospects. Trends Parasitol 19:488–494
Fast B, Kremp K, Boshart M, Steverding D (1999) Iron-dependent regulation of transferrin receptor expression in Trypanosoma brucei. Biochem J 342:691–696
Grab DJ, Wells CW, Shaw MK, Webster P, Russo DCW (1992) Endocytosed transferrin in African trypanosomes is delivered to lysosomes and may not be recycled. Eur J Cell Biol 59:398–404
Hirumi H, Hirumi K, Doyle JJ, Cross GAM (1980) In vitro cloning of animal-infective bloodstream forms of Trypanosoma brucei. Parasitology 80:371–382
Huber W, Koella JC (1993) A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop 55:257–261
Kristjanson PM, Swallow BM, Rowlands GJ, Kruska RL, de Leeuw PN (1999) Measuring the costs of African animal trypanosomosis, the potential benefits of control and returns to research. Agr Sys 59:79–98
Matovu E, Seebeck T, Enyaru JCK, Kaminsky R (2001) Drug resistance in Trypanosoma brucei spp., the causative agents of sleeping sickness in man and nagana in cattle. Microbes Infect 3:763–770
Nkemngu NJ, Grande R, Hansell E, McKerrow JH, Caffrey CR, Steverding D (2003) Improved trypanocidal activities of cathepsin L inhibitors. Int J Antimicrob Agents 22:155–159
Petray P, Castaños-Velez E, Grinstein A, Orn A, Rottenberg ME (1995) Role of nitric oxide in resistance and histopathology during experimental infection with Trypanosoma cruzi. Immunol Lett 47:121–126
Rockett KA, Awburn MM, Cowden WB, Clark IA (1991) Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun 59:3280–3283
Ross CA, Sutherland DV (1997) Drug resistance in trypamosomatids. In: Hide G, Mottram JC, Coombs GH, Holmes PH (eds) Trypanosomiasis and leishmaniasis: biology and control. CAB International, Wallingford, pp 259–269
Salvati L, Mattu M, Colasanti M, Scalone A, Venturini G, Gradoni L, Ascenzi P (2001) NO donors inhibit Leishmania infantum cysteine proteinase activity. Biochim Biophys Acta 1545:357–366
Scory S, Caffrey CR, Stierhof YD, Ruppel A, Steverding D (1999) Trypanosoma brucei: killing of bloodstream forms in vitro and in vivo by the cysteine proteinase inhibitor Z-Phe-Ala-CHN2. Exp Parasitol 91:327–333
Steverding D (2008) The history of African trypanosomiasis. Parasit Vectors 1:3
Steverding D, Stierhof YD, Fuchs H, Tauber R, Overath P (1995) Transferrin-binding protein complex is the receptor for transferrin uptake in Trypanosoma brucei. J Cell Biol 131:1173–1182
Steverding D, Caffrey CR, Sajid M (2006) Cysteine proteinase inhibitors as therapy for parasitic diseases: advances in inhibitor design. Mini Rev Med Chem 6:1025–1032
Venturini G, Colasanti M, Salvati L, Gradoni L, Ascenzi P (2000a) Nitric oxide inhibits falcipain, the Plasmodium falciparum trophozoite cysteine protease. Biochem Biophys Res Commun 267:190–193
Venturini G, Salvati L, Muolo M, Colasanti M, Gradoni L, Ascenzi P (2000b) Nitric oxide inhibits cruzipain, the major papain-like cysteine proteinase from Trypanosoma cruzi. Biochem Biophys Res Commun 270:437–441
Vespa GN, Cunha FQ, Silva JS (1994) Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasites in vitro. Infect Immun 62:5177–5182
Vincendeau P, Daulouède S, Veyret B, Darde ML, Bouteille B, Lemesre JL (1992) Nitric oxide-mediated cytostatic activity on Tyrpanosoma brucei gambiense and Trypanosoma brucei brucei. Exp Parasitol 75:353–360
WHO (2006) African trypanosomiasis (sleeping sickness). World Health Organ Fact Sheet 259 [http://www.who.int/mediacentre/factsheets/fs259/en/]
Acknowledgements
We thank Dr Conor Caffrey for rhodesain. This work was supported by the John & Pamela Salter Charitable Trust (RCN.298317).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steverding, D., Wang, X. & Sexton, D.W. The trypanocidal effect of NO-releasing agents is not due to inhibition of the major cysteine proteinase in Trypanosoma brucei . Parasitol Res 105, 1333–1338 (2009). https://doi.org/10.1007/s00436-009-1559-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1559-x