Abstract
Colitis-associated colorectal cancer has been a hot topic in public health issues worldwide. Numerous studies have demonstrated the significance of myeloid-derived suppressor cells (MDSCs) in the progression of this ailment, but the specific mechanism of their role in the transformation of inflammation to cancer is unclear, and potential therapies targeting MDSC are also unclear. This paper outlines the possible involvement of MDSC to the development of colitis-associated colorectal cancer. It also explores the immune and other relevant roles played by MDSC, and collates relevant targeted therapies against MDSC. In addition, current targeted therapies for colorectal cancer are analyzed and summarized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer worldwide and one of the leading causes of cancer-related deaths. With economic development and lifestyle changes, its incidence has been increasing year by year (Morgan et al. 2023), and studies have shown that the incidence of CRC has increased in people under 50 years of age in the past few decades (Spaander et al. 2023), with a shift towards diagnosis at a younger and more advanced stage (Siegel et al. 2023), as the population continues to age, the global cancer burden is expected to increase, making it a global public health problem that should not be underestimated. Previous studies have shown that people with inflammatory bowel disease (IBD) have an increased chance of developing colorectal cancer over time, the development of colorectal cancer, particularly colitis-associated colorectal cancer, is strongly associated with a cumulative inflammatory burden (Yvellez et al. 2021). Recent studies have shown that MDSCs in the immune system have a major impact on colorectal cancer progression, and together with many other immune cells, they jointly stimulate the proliferation of tumor cells, and participate in local angiogenesis and distal tumor metastasis.
In inflammatory bowel disease (IBD), intestinal inflammation and massive infiltration of bone marrow and lymphocytes are the main pathological features. It has been demonstrated that dendritic cells (DCs) and macrophages play a crucial role in controlling the development of pro-inflammatory lymphocytes, such as helper T cells and Th17 cells, within the intestines of individuals suffering from inflammatory bowel disease, and that pro-inflammatory lymphocytes further attract myeloid cells, including MDSC, into localized inflamed intestinal tissues. Currently, it has been found that MDSC infiltration is observed in animal models of CAC and patients with CAC, and these myeloid cells play a crucial role in facilitating the progression of IBD to CAC. Moreover, investigating the regulatory network of MDSCs in the development of CAC can help to explore new anti-tumor immunotherapeutic regimens for CAC targeting MDSCs, and enhancing the therapeutic effect of anti-CAC treatment is a novel approach. However, the mechanisms by which MDSCs control the progression of IBD to CAC remain largely unexplored. Here, we summaries the potential mechanisms of action of MDSC in the development of CAC from inflammatory bowel disease at this stage with other roles such as immune pathways, cytokines, and intestinal flora, and the potential ways in which MDSCs impact intestinal epithelial cells, resulting in the formation of low-grade and high-grade allopatric hyperplasia, and summarize the current therapeutic strategies for targeting MDSC-related CAC, as well as the research stages, existing findings and potential shortcomings of related studies, provide new insights into the specific ways in which MDSCs contribute to the progression of inflammatory bowel disease (IBD) to colorectal cancer (CAC) in the future, and also help to further explore the potential targets related to MDSCs for future CAC treatments, opening up new possibilities for effective therapeutic interventions.
MDSC and colitis-associated colorectal cancer
MDSCs play a critical role in the progression of colorectal cancer associated with colitis. MDSC are a diverse group of immature cells that originate from myeloid cells. In the bone marrow, hematopoietic progenitor cells normally undergo a process of differentiation into myeloid progenitor cells. These myeloid progenitor cells then continue to differentiate into granulocyte–macrophage precursors, followed by further differentiation into monocyte/dendritic cell precursors as well as mature myeloid cells, etc., and travel to secondary lymphoid organs, where they undergo further differentiation into monocytes and neutrophils to carry out specialized tasks (Wu et al. 2022b). MDSC can be broadly classified into polymorphonuclear cells (PMN-MDSC) and monocytes (M-MDSC), which share phenotypic and morphological similarities with neutrophils and monocytes, respectively. The myeloid cell markers CD33 + , CD11b + , HLA-DR low/- and Lin- can be used to identify human MDSC, which are divided into two main subgroups, the M-MDSC (CD33 + CD11b + CD14 + CD15- HLA-DR low) subgroup and the PMN-MDSC (CD33 + CD11b + CD14- CD15 + HLA-DR-) subpopulation (Gabrilovich 2017). However, under conditions of chronic inflammation or tumor, due to the abnormal proliferation of bone marrow, a variety of pro-inflammatory factors may be produced, interfering with the normal maturation of myeloid cells and lead to an increase in the number of immature myeloid cells, and these heterogeneous populations, which have similar physical characteristics to monocytes or granulocytes but whose specific surface molecular signatures differ from those of monocytes or granulocytes are collectively referred to as MDSCs, whose main feature is the potent immune-suppressing function under pathological conditions such as inflammation or tumor. MDSCs can exert immunosuppressive effects through a variety of pathways and mechanisms, including the upregulation of nitric oxide synthase (iNOS), arginase-1 (ARG-1), reactive oxygen species (ROS), etc. to inhibit lymphocytes, and indirectly inhibiting the body’s immune response by suppressing regulatory T cells (Tregs). Recent research has shown a strong link between MDSC expression and the advancement of CAC during the transition from colitis to CAC using clinical specimens and mouse animal models, and MDSCs have become a key target in current oncology research due to their abundant presence in the tumor microenvironment (Chen et al. 2022a, b). MDSC infiltration is often seen at sites of inflammation in patients with chronic inflammatory diseases and tumor, relevant studies have shown that certain pro-inflammatory mediators present in the microenvironment, like IL-6, contribute to the promotion of MDSC accumulation in the pathological state of chronic inflammation and malignancy (Laws et al. 2023). Prostaglandin E2 (PGE2) is a significant lipid mediator that produced in the sustained inflammatory response, also further recruits MDSC, which in chronic inflammation further produce S100A8/A9 proteins, these calcium-binding proteins are mainly secreted by neutrophils and activated monocytes, and interestingly, these proteins in the inflammatory microenvironment in the inflammatory microenvironment further recruiting more MDSC causing further MDSC accumulation, the inflammatory microenvironment can also further increase the generation of reactive oxygen species (ROS) and pro-angiogenic factors like vascular endothelial growth factor (VEGF), which can contribute to MDSC accumulation and immunosuppressive activity, in addition, the tumor microenvironment and persistent inflammatory stimuli can also lead to an additional boost in the production of tumor necrosis factor alpha (TNF-α) by tumor cells, which also further recruits MDSC and enhances MDSC-associated immunosuppressive activity (Wang et al. 2023a, b, c, d, e). Multiple research studies have consistently shown that reducing MDSCs can effectively slow down tumor progression and lead to anti-tumor effects, and all these evidences suggest that MDSCs play unique and crucial roles in the progression of inflammatory bowel disease to CAC development (Krishnamoorthy et al. 2021; Liao et al. 2019; Wang et al. 2023e).
Potential role of MDSC
The primary feature of MDSC is their capacity to inhibit the immune response. Each subtype of MDSC has different characteristics that affect their ability to regulate various components of the immune response. For example, PMN-MDSC primarily utilizes prostaglandin E2 (PGE2), arginase 1 and ROS for immunosuppression, whereas M-MDSC utilizes NO, IL-10, TGF-β, and PD-L1 for the same purpose (Youn et al. 2008).
Immune pathway related
STAT3 pathway
In the tumor microenvironment, MDSC are activated by multiple mechanisms. The crucial role is played by the transcription factor STAT3 (Zhang et al. 2024). STAT3 is an important oncogenic transcription factor, and phosphorylation of Bcl-2 regulates the expression of genes that prevent apoptosis, angiogenesis-related (e.g., VEGF), and some specific factors (e.g., S100A9), which are all relevant to colitis-associated colorectal cancer tumor invasion, metastasis and prognosis.
MDSC express receptors for S100A8 and S100A9, Wang et al. (2020) used CRISPR CAS9 to knock down specific immunosuppressive factors in a mouse tumor model and found that STAT3 has a significant role in controlling the differentiation of MDSCs. Additionally, STAT3 was found to increase the expression of S100A8/9 proteins, which promotes the clustering of MDSCs in the tumor microenvironment (TME). This clustering ultimately leads to the activation and multiplication of MDSCs (Wang et al. 2023a, b, c, d, e). Using single-cell cytokine profiling for immunoassay, G. Qin et al. found that MDSCs were significantly expressed in both clinical samples and mouse models of tumors, Additionally, they observed that specific factors like GM-CSF, G-CSF, IL-6, VEGF, etc., amplified the quantity of MDSCs in the tumor microenvironment and impeded their subsequent differentiation, and these factors further activated the JAK/STAT signaling pathway to stimulate myeloid cell production and promote MDSC expansion (Qin et al. 2023). Wu et al. found that the only inhibitory receptor in the Fcγ receptor family, FcγRIIB, was expressed in tumor-infiltrating MDSCs by constructing a mouse model of Fc γ receptor IIB receptor deficiency in the Immunoglobulin (Ig) Fc region, and using adoptive cell transfer, mRNA sequencing, and flow cytometry analysis to discover that the only inhibitory receptor in the Fcγ receptor family was responsible for upregulating the presence of tumor-infiltrating MDSCs. This receptor also played a role in promoting the production of MDSCs by hematopoietic progenitor cells through the Stat3 signaling pathway, thereby bolstering the immunosuppressive functions of MDSCs, resulting in tumor immune evasion (Wu et al. 2022a, b). In addition, there are also studies on multifunctional spore shell nanoparticles (CN) encapsulated on the surface of probiotics, and it was found that CN-encapsulated probiotics were successful in treating inflammatory bowel disease and preventing colorectal cancer. The activation of STAT3 was discovered to enhance the functionality of epithelial cells and prevent the death of intestinal epithelial cells (IECs). This, in turn, has an impact on the integrity of the intestinal barrier and the stability of the intestinal microenvironment, and that blocking the IL-6 -STAT3 signaling pathway through CN-encapsulated probiotics could prevent colitis-related colorectal cancer. Blocking the IL-6-STAT3 signaling pathway by CN-encapsulated probiotics prevented the prevention of colitis-associated colorectal cancer was achieved, which further confirmed the pathogenic role of STAT3 in colitis-associated colorectal cancer (Song et al. 2021).
NF-κB pathway
Studies have confirmed that IL-1β activates the NF-κB signaling pathway to promote MDSCs mobilization and proliferation. As a result, the progression of gastritis and gastric cancer is promoted (Tu et al. 2008). One of the functions of activating the NF-κB signaling pathway is to promote the mobilization of MDSCs, which in turn promotes colorectal tumorigenesis associated with ulcerative colitis. This study found that the glycoheterotrophic enzyme fructose 1,6-bisphosphatase 1 (FBP1) can directly interact with IκBα, thereby inhibiting NF-κB activation and suppressing colorectal tumorigenesis (Zhu et al. 2023). When VEGF is highly expressed in the TME, it can activate the NF-κB pathway. As a result of this activation, FLT3L expression is suppressed, which promotes the abnormal differentiation of myeloid progenitor cells into MDSCs. The NF-κB family member c-Rel was utilized in the study, and the use of a small-molecule inhibitor targeting c-Rel led to a notable reduction in the tumor-suppressing capabilities of MDSCs, ultimately resulting in the inhibition of tumor growth (Li et al. 2020). The regulation of apoptosis and proliferation of IECs is significantly influenced by the NF-κB signaling pathway. Furthermore, it plays a crucial role in preserving the integrity of the intestinal barrier and promoting the body’s defense against pathogens. The NF-κB signaling pathway is essential for preserving the integrity of the intestinal epithelium and ensuring a balanced immune response in the intestine, when NF-κB is deficient, this leads to the death of IECs, decreased production of antimicrobial peptides, and the movement of bacteria into the mucosa. These events contribute to the progression of inflammatory bowel cancer (Nenci et al. 2007). The close relationship between the NF-κB signaling pathway and the formation, recruitment, and activity of MDSCs is clearly evident, and is a key factor in colitis-associated colorectal cancer.
COX-2/PGE2 pathway
MDSC inhibitory activity is significantly influenced by prostaglandins, particularly prostaglandin E2 (PGE2). PGE2 has been linked to the promotion of tumor growth, angiogenesis, and the suppression of the immune system.
Relevant studies have shown that PGE2 inhibits immunity by recruiting MDSC, among others, and contributes to tumor angiogenesis. In the early stage of tumor formation, COX-2/PGE2 pathway can drive the inflammatory microenvironment, which promotes downstream signaling, and consequently, tumor progression in an irreversible direction. In the in vivo environment of colitis-associated colorectal cancer patients, overexpression of COX-2 leads to increased production of vascular growth factors. This, in turn, stimulates endothelial cell migration, leading to increased invasiveness and the spread of cancer cells is inhibited by reducing the Bcl-2 gene expression and decreasing apoptosis, etc. Thus, increased expression of COX-2 is associated with the development, progression and spread of colorectal cancer. CXCL1 is a pro-angiogenic CXCL1 is a pro-angiogenic chemokine, and PGE2 can induce CXCL1 expression, increase MDSC infiltration in colitis-associated colorectal cancer mouse models, promote in vivo tumor growth and increase tumor micro vessel formation. In recent years, there is strong evidence indicating that the prolonged use of NSAIDs significantly decreases the likelihood of developing colorectal cancer (CRC) (Friis et al. 2015), an interesting finding that has led to a reexamination of the "miracle drug" aspirin and its target cyclooxygenase (COX), COX is responsible for metabolizing arachidonic acid, released from membrane phospholipids by phospholipase A2 (PLA2), into prostaglandin H2 (PGH2) via specific isoenzymes and TXX2, an enzyme that is a key component of prostaglandin H2 (PGH2). COX has the ability to break down arachidonic acid, which is released from membrane phospholipids by phospholipase A2 (PLA2), and convert it into prostaglandin H2 (PGH2), which is converted by specific isomerase and TXA synthase into different prostaglandins (such as PGE2, PGD2, PGF2α, PGI2, etc.) and TXA2, and COX has two isozymes, COX1 and COX2, with COX-1 being responsible for the maintenance of tissue homeostasis during regular physiological conditions. COX-1 is primarily responsible for maintaining the necessary basal levels of prostaglandins for tissue balance in normal physiological situations. On the other hand, COX-2 is predominantly found in inflammatory cells (Ye et al. 2020). Recent research has demonstrated that elevated levels of COX-2 in colon tumor cells lead to an increased production of PGH2 from arachidonic acid, and therefore the expression of PGE2 is increased in the microenvironment. PGE2 can enhance the progression of colorectal cancer by inducing apoptosis, promoting cell proliferation, stimulating angiogenesis, and facilitating metastasis (Karpisheh et al. 2019). The expression of MYO10 (signaling axis) is decreased when COX-2 is knocked down, resulting in a decrease in the migratory and invasive capabilities of colon cancer tumor cells (Liu et al. 2023a, b). At the same time, COX-2 inhibitors, resist epithelial-mesenchymal transition (EMT) alterations in CRC cell lines (This is also a key process in cancer cell metastasis, where epithelial cells undergo a transformation from their original characteristics to mesenchymal characteristics, resulting in enhanced motility and migratory abilities). It has also been shown that co-targeting COX2 with BRAF + EGFR durably inhibits tumor growth capacity in patient-derived tumor xenograft models (Ruiz-Saenz et al. 2023). COX2 inhibition represents a strategy that could overcome associated CRC treatment resistance. Currently, a variety of COX-2 small molecule inhibitors, such as rofecoxib, have entered the phase III clinic (NCT00031863), and other small molecule inhibitors, such as imrecoxib (ChiCTR2100051644), lumiracoxib (NCT00170898), and meloxicam (NCT01886872), have also entered the clinic and have been successfully marketed. NCT01886872) have also entered the clinic and been successfully marketed. It is believed that with the development of COX-2 inhibitors with higher safety and specificity in the future, it will be more helpful in preventing and controlling the development of tumors.
Cytokine-related
New research has indicated that specific inflammatory cytokines and chemokines have the ability to promote the proliferation of MDSCs. On one side, they are withholding crucial amino acids from T cells, induce oxidative stress, and are involved in regulating the functions of Tregs and T helper (Th)17 cells, etc. On the other hand, They stimulate MDSCs to release reactive oxygen radicals (ROS), inducible nitric oxide synthase (iNOS), and arginase 1 (Arg-1), which suppress T cell activity and promote T cell apoptosis (Ma et al. 2020). At the same time, an imbalance between pro-inflammatory and anti-inflammatory cytokines can fuel the progression of IBD, leading to the development of colitis-associated colorectal cancer.
IL-10: A cytokine known to suppress inflammation. Chronic inflammation in ulcerative colitis contributes to the accumulation of high levels of MDSCs in the colon, and in turn, high levels of MDSCs produce higher levels of IL-10, but the function of IL-10 is altered in this environment IL-10 instead activates STAT3, which results in increased expression of two genes, DNMT1 and DNMT3b-, contributing to the silencing of a tumor suppressor (Ibrahim et al. 2018). Leading to possible IEC heteroplasia, driving a higher incidence of inflammatory bowel cancer.
IL-6: The progression of colitis-associated colorectal cancer was also significantly affected by IL-6. It has the ability to activate Janus kinase (JAK) and induce phosphorylation of the transcription factor STAT3 downstream, thus promoting the development of cancer. Apart from the JAK/STAT3 pathway, IL-6 can also exacerbate the intestinal inflammatory response in patients and promote colitis-associated colorectal cancer by regulation of Th17 and Treg cell proliferation and function (Wang et al. 2023a, b, c, d, e). IL-6 produced by MDSCs can hinder the development and activity of CD4( +) T cells, ultimately promoting tumor development (Tsukamoto et al. 2013). It has been shown that blocking IL-6 enhances the therapeutic effect of immunotherapy by inducing and recruiting higher levels of CD4 + /CD8 + effector T cell production and recruitment in the tumor microenvironment (Hailemichael et al. 2022).
TGF-β: It can exert its anticancer effects through antiproliferative, pro-apoptotic and inhibiting the production of inflammatory factors with pro-tumor activity. However, TGF-β also modulates the immunosuppressive effects of MDSCs and hinders the ability of immune cells to fight against tumors, and also promotes epithelial-mesenchymal transition (EMT), thus accelerating tumor metastasis (Yang et al. 2008).
TNF-a: It is an important pro-inflammatory factor that plays a crucial role in the advancement and growth of colorectal cancer linked to colitis. This inflammatory mechanism is primarily responsible for the sustained activation of the NF-κB signaling pathway. It can also stimulate colitis-associated colorectal cancer production by damaging DNA. It has been shown that TNF-α indirectly mediates the effects of ROS on the stem cell microenvironment and plays an important role in epithelial cells (Hsu et al. 2022). Blocking TNF can attenuate colorectal cancer by altering the composition and activity of the microbiota (Yang et al. 2020).
Other relevant avenues
In the corresponding environment, MDSC release reactive oxygen radicals (ROS), etc. The large amount of reactive oxygen ROS etc. produced can cause oxidative stress. Studies have shown that oxidative stress induces the misvocalization of nuclear RNA or the DNA-binding protein TDP-43, which further leads to the overaccumulation of the R-loop (i.e., DNA in the form of an RNA heterozygous strand structure formed by DNA and RNA), which in turn triggers DNA damage and instability in the genome, resulting in the over-activation of the key enzyme for DNA repair, PARP1. This further trigger depletion of the coenzyme NAD + and ATP deficiency, which promotes the onset of mitochondria-dependent necrotic apoptosis in intestinal epithelial cells, which in turn drives the onset of spontaneous intestinal inflammation (Yang et al. 2024). In addition, excess ROS can cause damage to intestinal cells. This damage occurs through a variety of mechanisms, including induction of DNA mutations, impairment of protein function, alteration of epithelial permeability, and disruption of the intestinal epithelial barrier. These effects ultimately lead to cancer development and the proliferation of tumor cells (Wang et al. 2023a, b, c, d, e; Zhang et al. 2023). Thus, inflammation-induced oxidative stress is clearly also important in the progression of colitis-associated colorectal cancer.
Several studies have shown that the metabolism of intestinal flora can also be closely related to the progression of colitis-associated colorectal cancer. Associated microbiota can induce aggregation of MDSCs and pro-inflammatory functions. For example, the presence of commensal Gram-negative gut bacteria can lead to the accumulation of MDSCs (Zhang et al. 2020). FadA adhesin produced by Clostridium nucleatum binds to E-calmodulin to produce pro-inflammatory factors and activates the TLR4/NF-κB signaling path way via lipopolysaccharide LPS to promote the recruitment of MDSCs to the infection site, this recruitment activates the Wnt/β-catenin signaling pathway, which further modulates the bacterial adhesion and invasion into the epithelial cells, ultimately, this process promotes the proliferation of colorectal cancer cells that Promotion of colorectal cancer (Yang et al. 2024). Enterotoxin-producing bacterium Enterobacteriaceae fragilis (ETBF) induces Th17 recruitment and inhibits T-cell proliferation, contributing to a pro-inflammatory milieu that favors the production and differentiation of pre-tumorigenic monocyte-derived cells (MDSCs), as a consequence, the chances of developing colorectal cancer may increase (Thiele Orberg et al. 2017).
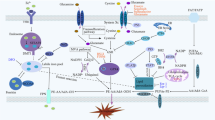
Figure 1 illustrates the involvement of MDSC in colitis-associated colorectal cancer. However, the specific role of MDSC in the advancement of colitis-associated colorectal cancer is not fully understood, and more research is necessary to gain a comprehensive understanding of how MDSC contributes to the formation of inflammatory bowel cancer.
MDSC exerts relevant immunosuppressive functions in the inflammatory microenvironment and influences tumor development. Associated chemokines (CSF, VEGF, CXCLx, etc.) recruit MDSC in the inflammatory microenvironment; MDSC play an immunosuppressive role by inhibiting T-cell-associated functions; MDSC promote the involvement of Treg cells in the associated suppression of anti-tumor immune responses; MDSC promote epithelial-mesenchymal transition (EMT), tumor angiogenesis, and enhancement of tumor cell stemness
Effects of MDSC on intestinal epithelial cells
A sustained and persistent inflammatory response is critical to the development of colorectal cancer associated with colitis. This inflammatory response plays a role in every stage of tumor formation and development. Colitis significantly increases the risk of colorectal cancer due to the long-term accumulation of inflammation. Chronic inflammation and increased epithelial cell renewal will lead to the formation of both low-grade and high-grade heterogeneous proliferation of intestinal epithelial cells, which may promote the further transformation of IBD to colitis-associated colorectal cancer, and the poor prognosis of patients who progress from inflammatory bowel disease to colorectal cancer has a higher mortality rate. Recent studies have shown that patients with inflammatory bowel disease, MDSC promotes the proliferation of intestinal epithelial cells, triggers heteroplasia of intestinal epithelial cells, and enhances the ability of tumor cells to maintain stem cell properties, thus contributing to irreversible cancerous transformation of intestinal epithelial cells.
Promotes proliferation of IECs
Relevant studies have shown that under conditions of inflammatory bowel disease, MDSC-derived IL-6 activates STAT3 and stimulates intestinal epithelial cell IEC hyperproliferation. Invasiveness, proliferation and stemness of human colon cancer cells are closely related to Stat3 activation (Liu et al. 2022). Furthermore, it has been shown that elimination of Stat3 from epithelial cells greatly hinders the development of colitis-associated colorectal cancer. This is achieved by inhibiting cell proliferation and promoting apoptosis. It has also been shown that removal of cyclic guanosine-adenylate synthase (cGAS) promotes the recruitment and activation of MDSCs in the colon, and proliferation of intestinal epithelial cells and increased permeability of the intestinal barrier. It may promote colonic inflammation and cancer development (Hu et al. 2021).
Elicits heterogeneous hyperplasia in the IECs
MDSC promote tumor progression by promoting chronic inflammation, facilitating angiogenesis, and establishing a tumor microenvironment that suppresses the immune system. It has been shown that PAR2 deficiency in MDSC directly enhances its immunosuppressive activity by promoting STAT3-mediated reactive oxygen species production, contributing to IEC heteroproliferation (Ke et al. 2020). In the microenvironment of inflammatory bowel disease, MDSC-derived ROS may cause IEC heteroproliferation by damaging IEC DNA, inducing DNA alterations in epithelial cells and inhibiting their repair. Under inflammatory conditions, IL-6-activated STAT3 also plays an important role in IEC heteroplasia (Grivennikov et al. 2009).
Enhancement of tumor cell stemness
In an inflammatory environment, MDSCs can also promote colitis-associated colorectal cancer progression by promoting stemness in colon cancer cells. There have been reports indicating that netrin-1 inhibits PMN-MDSC recruitment and promotes tumor cell stemness, which is closely related to drug resistance and cancer recurrence after chemotherapy or immunotherapy (Ducarouge et al. 2023). MDSC-associated PGE2 induces iPSC in mice to acquire the characteristics of cancer stem cells (CSCs) through the PI3K/Akt axis. Thus, it is clear that PGE2 also contributes to the promotion of cancer cell stemness that leads to colitis-associated colorectal cancer (Minematsu et al. 2022).
Targeting MDSC-related therapeutic strategies
MDSCs consistently recruited in the tumor microenvironment have the ability to inhibit T cell proliferation and impair their function. Therefore, targeting MDSCs is a potential strategy for corresponding tumor therapy. We summarized the current relevant studies targeting MDSC therapy as shown in Table 1.
Depletes MDSC
Chemotherapeutic agents such as gemcitabine, cyclic tetrazolamide (CTX), 5-fluorouracil, oxaliplatin, paclitaxel, and others have the potential to improve the effectiveness of immunotherapy in combating tumors. They achieve this by targeting the immunosuppressive cells present in the tumor microenvironment (Gürlevik et al. 2016). Gemcitabine, as a nucleoside analogue, is now widely used in the first-line treatment of various solid tumors. Gemcitabine can directly kill tumor cells and down reduce the quantity and activity of MDSCs, and enhance T-cell-mediated immune responses against tumors (Jiang et al. 2023). Relevant studies have shown that chemotherapeutic agents such as CTX can decrease the expression level of multidrug and toxin extrusion transporter protein ABCB1 in Treg cells, thereby depleting Treg and MDSC cells and inhibiting tumor growth (Galluzzi et al. 2020). Chemotherapeutic drugs such as fluorouracil (5-FU) has been recognized as an important drug for colorectal cancer since it was applied in the clinic in 1957. It can block the synthesis of the nucleoside thymidine, which is essential for DNA replication, and the relevant studies have found that MDSCs in several mouse tumor models display lower levels of the enzyme targeted by 5-FU. Moreover, cells with reduced thymidine synthase expression are highly vulnerable to cell death caused by 5-FU. Due to their low expression of thymidylate synthase, cells are highly susceptible to cell death induced by 5-FU. As a result, 5-FU can selectively eliminate MDSCs by inducing apoptosis, and the administration of 5-FU treatment significantly reduces tumor growth rate in mouse models and achieves therapeutic efficacy (Kim et al. 2021). A randomized controlled trial conducted on colon cancer patients in phase II has also shown that 5FU can effectively combat tumors by specifically targeting and eliminating MDSC. Studies conducted both in vitro and in vivo have demonstrated that 5FU has strong cytotoxicity against MDSC, thereby mediating immune tolerance. The 5FU-induced reduction in MDSC also further enhances the production of interferon-Y (IFN-Y) by CD8( +) T cells, which contributes to the promotion of anti-tumor responses (Wang et al. 2023a, b, c, d, e).
Tyrosine kinase inhibitors such as sunitinib can directly target the amplification signaling pathway of MDSC to achieve the purpose of MDSC depletion. Sunitinib is a targeted therapeutic drug that can inhibit the activity of multiple receptor tyrosine kinases, and relevant clinical studies have demonstrated that sunitinib inhibits the growth and spread of cancer cells, showing obvious anti-tumor activity and safety (Heinrich et al. 2024; Vallilas et al. 2021). A recent study reported that two multi-targeted tyrosine kinase inhibitors, carbonatitic or celecoxib, were able to reduce MDSC levels in a mouse model of squamous cell carcinoma (Huang et al. 2020). Additionally, there are studies that have assessed the effectiveness and safety of combining the tyrosine kinase inhibitor cabozantinib with a PD-L1 inhibitor in patients with advanced colorectal cancer within a specific colorectal cancer population. The results demonstrated that this treatment plan demonstrated significant effectiveness in fighting tumors and had tolerable side effects in patients with advanced colorectal cancer that did not respond to previous treatments (Saeed et al. 2024).
Depleting MDSCs continues to be a crucial approach for enhancing anti-tumor immunity, and as we uncover more MDSC-related targets, this strategy will become even more significant in the future, it will also surely provide new ideas for future therapeutic options.
Induces MDSC differentiation
To successfully decrease the quantity of MDSC in mouse tumor models and cancer patients, we can also achieve our desired effect by inducing differentiation of immature myeloid cells.
Vitamins such as vitamin D and vitamin E can induce MDSC cell differentiation. Relevant studies have shown that 1,25-dihydroxyvitamin D3 plays a crucial role in controlling cell growth and differentiation, and recent research has demonstrated that 1,25-dihydroxyvitamin D3 has the ability to transform normal human myeloid cells into macrophages and monocytes, vitamin E enhances the immune response by reducing the production of ROS and NO and reducing the presence of immature MDSCs (O'Mahony et al. 2023). ATRA, also known as trans-retinoic acid, is a substance derived from vitamin A. It is used in the production of myeloid cells. The transformation of MDSCs into fully developed myeloid cells is significantly influenced by it. Several studies have demonstrated the ability of ATRA to activate the ERK1/2 kinase pathway and then upregulate glutathione, further scavenging ROS, resulting in a decrease in the amount of ROS in MDSCs. After administering ATRA treatment, the number of MDSCs in a mouse model of tumor showed a significant decrease, and MDSCs were stimulated to differentiate into dendritic cells and macrophages, which transformed their immune-suppressing function into an immunogenic one, greatly improving the anti-tumor effect. greatly improved the anti-tumor effect (Hengesbach and Hoag 2004). 24 patients with advanced melanoma participated in a phase 2 clinical trial (NCT03200847) to evaluate the effectiveness of combining ATRA and Pembrolizumab, it was demonstrated that the ATRA combination therapy was well-tolerated and safe in patients with melanoma. Additionally, it effectively decreased the proportion of MDSCs while increasing the proportion of myeloid cells, and that the majority of the patients achieved significant symptomatic relief. In other words, patients treated with ATRA had reduced levels of MDSCs and promoted MDSC differentiation, and this alteration in differentiation subsequently decreased the immunosuppressive effects of MDSCs, translating into enhanced immunotherapy efficacy and safe and effective treatment (Olson and Luke 2023).
Also some researchers have found in mouse tumor models that diosgenin not only triggers apoptosis in MDSCs, but also promotes their transformation into M1-like macrophages while reducing the number of M-MDSC cells. This discovery weakens the development of colitis-associated colon cancer, making it a promising natural option for effectively preventing CAC. We believe that in the future, we can explore more relevant drugs for inducing MDSC differentiation, which can help the treatment of CAC (Xun et al. 2023).
Inhibits MDSC recruitment and migration
Under pathological conditions such as inflammation, tumors, etc., this leads to the recruitment and migration of MDSCs, which then exert their immunosuppressive function. Therefore, there are many studies to stop this process.
VEGF inhibitor vascular endothelial growth factor (VEGF) is a distinctive growth factor that specifically targets vascular endothelial cells, stimulating their growth and enhancing their functionality. It is essential for increasing vascular permeability, breaking down the extracellular matrix, supporting endothelial cell motility and growth, and stimulating the growth of fresh blood vessels via the process of angiogenesis. Tumor cells are the main source of VEGF and play a role in TME. VEGF, in turn, Stimulates the growth of fresh blood vessels (angiogenesis) and induces MDSCs to enter the tumor. To address this problem, monoclonal antibodies (mAb) have been developed that specifically target VEGF. These mAb have shown promising results in inhibiting tumor growth in mouse cancer models and in human patients (Shojaei et al. 2007). Preclinical and clinical studies have shown that bevacizumab, a recombinant human monoclonal antibody targeting vascular endothelial growth factor, is effective in reducing intratumorally MDSCs. During a Phase 2 clinical trial (NCT01730950), the utilization of bevacizumab demonstrated a significant improvement in progression-free survival (PFS) in patients with recurrent glioblastoma (GBM). This treatment effectively reduced the occurrence of MDSC and prevented MDSC recruitment and movement, resulting in clinically significant efficacy. A trial has shown a reduction in circulating MDSCs after combination therapy with bevacizumab and 5-fluorouracil, oxaliplatin in patients with colorectal cancer (Limagne et al. 2016a, b). Perhaps we can explore more relevant combination therapy options in the near future.
HIF-1α inhibitor: The increased expression of HIF-1α in the hypoxic environment of TME can lead to an increase in glycolytic enzymes and lactate transporter proteins in MDSCs as a way to promote MDSC differentiation and proliferation. Moreover, HIF-1α can also improve mitochondrial respiratory function through the PI3K/AKT and JAK2/STAT3 pathways, as well as attenuate cellular oxidative stress and reduce ROS production, thus alleviating MDSC cell injury. Meanwhile, the acidic environment formed by hypoxia promotes MDSC proliferation and enhances the inhibitory function of MDSC on T cells through HIF-1α. HIF1α hypoxia-inducible factor. Stable expression under hypoxic conditions. Plays an important role in MDSC accumulation. Therefore, MDSC recruitment in TME can be reduced by HIF1α inhibitors. Against hypoxia, a phase 1/2 clinical study (NCT01522872) involving TH-302 (efaproxiral), a hypoxia-activated prodrug, demonstrated extended survival in patients with myeloma, combating the effects of hypoxia (Laubach et al. 2019). In mouse kidney and mammary tumor models, researchers found that a nano-enzyme called Zr-CeO exhibited promising results in diminishing the recruitment of MDSCs, thereby enhancing the efficacy of PD-1 inhibitors in combating tumors (Mo et al. 2023).
Calcium-binding proteins S100A8 and S100A9, it plays a crucial role in the accumulation of MDSCs. Reduction of S100A8/A9 reduces MDSC accumulation in several mouse tumor models. Tiquinamide has been proven to decrease the infiltration and accumulation of MDSCs, with S100A9 as one of the targets. In a phase II clinical trial, results showed that administration of guanosine amide was effective in reducing the infiltration and accumulation of MDSCs, of which S100A9 is a specific target. This significantly slowed disease progression in metastatic CRPC. Furthermore, tiquinamide demonstrated enhanced progression-free survival rates in metastatic refractory prostate cancer (mCRPC) (Mehta and Armstrong 2016). The results indicate that the aggregation of MDSCs is significantly influenced by the involvement of S100A8/A9.
Chemokine receptors, it plays a key role in directing MDSCs to the tumor site. MDSCs are mainly identified by their expression of the chemokine receptor CCR2 and are attracted to tumors that produce the chemokines CCL2 and CCL5.
Research has demonstrated that blocking the CCL2/CCR2 axis with CCR2 antagonists, either on their own or in conjunction with other compounds, reduces the presence of MDSC in tumors and enhances tumor condition in preclinical mouse models (Nywening et al. 2016). The CCR2 inhibitor PF-04136309 has been shown to improve the survival of pancreatic cancer patients in combination with FOLFIRINOX, improves the chances of survival for individuals diagnosed with pancreatic cancer, demonstrating an improved antitumor response (NCT01413022).
In conclusion, these studies suggest that drugs targeting CCR2 can effectively decrease the levels of MDSCs and enhance the survival rates of individuals with cancer.
The small molecule inhibitor SX-682, which targets CXCR1 and CXCR2, effectively reduces the accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs). When used with checkpoint inhibitors, it has demonstrated promising results in improving the body’s immune response against tumors.
Studies have indicated that the combination of the CCR5 antagonist maraviroc with pembrolizumab is feasible in metastatic colorectal cancer (NCT03274804) (Liu et al. 2023a, b). It is uncertain whether these drugs will be successful in reducing the buildup of MDSCs and enhancing the immune system’s ability to fight tumors.
Targeting CSF1-R is also a method to block the migration of MDSCs to tumor locations. Binding of CSF1-R to ligand CSF1 stimulates the development and proliferation of myeloid cells. Studies performed in a mouse tumor model demonstrated that blockade of CSF-1R using a specific inhibitor (BLZ945) reduced the aggregation of MDSCs and shrunk tumor size. This highlights the important role of CSF-1R signaling in recruiting MDSCs (Mao et al. 2016).
Currently, a phase I clinical trial (NCT02880371) is in progress to assess the safety and initial effectiveness of the colony-stimulating factor-1 receptor-specific inhibitor ARRY-382 (PF-07265804). The trial is designed to evaluate the combination of ARRY-382 and pembrolizumab in advanced solid tumors (Johnson et al. 2022).
Modulates the immunosuppressive function of MDSC
Modulating the immunosuppressive function of MDSCs has been utilized as a therapeutic approach to enhance the function of T cells and other immune cells.
The researchers found that by blocking iron death in the mouse model, they were able to eliminate the inhibitory effect of PMN-MDSC on T cells. This led to a significant reduction in tumor growth. Moreover, the use of iron death inhibitors combined with anti-PD1 treatment exerted a better anti-tumor therapeutic effect (Kim et al. 2022). M-MDSC induced an increase in adenosine levels, and depletion of adenosine in TME using polyethylene glycolate adenosine deaminase (PEG-ADA) improved the efficacy of immune checkpoint inhibitor (ICI) therapy in mouse studies. This provides new ideas for overcoming resistance to ICI in cancer patients (Sarkar et al. 2023).
As previously described, the accumulation and growth of MDSCs in tumors are greatly affected by the STAT family of transcription factors. Among these factors, STAT3 plays a particularly vital role in this mechanism.
The STAT3 inhibitor Napabucasin significantly increased the survival of melanoma-bearing mice. This inhibitor successfully eliminated the immunosuppressive capacity of mouse MDSC and human M-MDSC (Bitsch et al. 2022). Experimental evidence from a Phase 1b clinical trial (NCT01563302) demonstrated that the STAT3 inhibitor AZD9150 (Danvatirsen) was able to reduce granulocyte MDSC levels. In addition, Danvatirsen alone or in combination with checkpoint inhibitors has shown encouraging results in reducing PMN-MDSC levels in diffuse B-cell lymphoma. Combination therapy with Danvatirsen and the anti-PDL1 monoclonal antibody Durvalumab has demonstrated encouraging outcomes in the treatment of individuals suffering from advanced solid tumors (NCT02983578) (Reilley et al. 2018).
TGF-β promotes MDSC expansion, differentiation and immunosuppressive functions. A TGF-βR inhibitor, LY3200882, was used in a clinical study (NCT02937272) in patients with solid tumors (Yap et al. 2021). As an anti-cancer therapy, inhibition of TGF-β signaling may affect cardiac development and function, which may be a major challenge in the study. Therefore, the development of anti-TGF-β combination therapy is necessary to enhance the clinical efficacy and reduce the toxicity.
Toll-like receptor (TLR) 7/8 small molecule agonists are strong activators of mature activation in antigen-presenting cells (APCs). In a study using mice to model colon cancer, the TLR 7/8 agonist resiquimod (R848) has been shown to reduce MDSCs within tumors and in the bloodstream. In addition, it inhibits the immunosuppressive capacity of MDSCs. In another study, the combination of oxaliplatin and the Toll-like receptor agonist R848 was found to disrupt the differentiation of myeloid-derived suppressor cells (MDSCs), thereby enhancing oxaliplatin resistance in colorectal cancer patients. In addition, this combination therapy improves the anti-tumor efficacy of oxaliplatin (Liu et al. 2020).
Histone deacetylase (HDAC) inhibitors are able to impair MDSC-mediated immunosuppression. It has been shown that when the HDAC 6 inhibitor ricolinostat was used in combination with the HDAC 1 inhibitor Entinostat, both MDSC populations were completely eliminated and tumor progression was delayed in several tumor models (Luke et al. 2023). However, the HDAC 6 inhibitor ricolinostat alone only reduced M-MDSC, failed to reduce PMN-MDSC and did not reduce tumor growth in the tumor models. A similar situation occurred with Entinostat alone (Hashimoto et al. 2020). Valproic acid is an HDAC inhibitor that has been shown to reduce tumor infiltration by MDSCs, attenuate immunosuppression caused by MDSCs, and improve the efficacy of anti-PDL1 immunotherapy (Xie et al. 2020). Entinostat combination therapy has shown positive response in advanced solid tumors, as reported in a phase 2 clinical trial (NCT 01928576).
In a clinical trial (NCT02903914), it was demonstrated that small molecule inhibitors of ARG-1 can reduce levels of iNOS and COX-2, while also regulating immunosuppressive functions in MDSC. The drug combination, which included the ARG-1 inhibitor CB-1158, was proven to be both safe and effective in patients with advanced and metastatic tumors.
A recent clinical study (NCT03043313) conducted on individuals diagnosed with colorectal cancer has shown promising results in the use of tucatinib, a small molecule HER2 inhibitor, in combination with trastuzumab. and was well tolerated by the participants (Strickler et al. 2023).
During a phase 3 clinical trial (NCT00843635), tadalafil treatment was found to be well tolerated and had a positive impact on patients with HNSCC by lowering MDSC levels. It was observed that higher doses of tadalafil had no relevant immunomodulatory activity, but intermediate doses were found to be most effective.
Targeted therapy related to colorectal cancer
Current targeted therapies for colorectal cancer
Looking at Table 2, we can see that although some phase I and small phase II clinical trials have achieved interesting results, phase II and III trials in a wider patient population have not shown convincing efficacy, and the treatment also faces the challenge of drug-related toxicity and the emergence of drug resistance. In the meantime, researchers are exploring combinations of drugs, exploring new targets, and refining targeted therapies in the future to enhance the quality of life and prognosis for patients with bowel cancer.
Challenges, opportunities, and possible future directions
It is undeniable that additional research is necessary in the future to tackle the challenges related to the development of MDSC in inflammatory bowel disease and its link to the initiation of colorectal cancer:
-
1. Firstly, the definition of MDSC itself is indeterminate, which hinders our study of it (Hegde et al. 2021). The definition of extant DSCs is inherently limiting. Only two categories are currently defined, M-MDSC and G-MDSC. but there are no known major regulators of MDSC properties and a range of MDSC-like phenotypes are observed in inflammation or cancer. Therefore, we need to develop a new research idea to refine the definition of MDSC.
-
2. Can MDSC be used as a biomarker of true response? It is not yet known and clinical trials are still needed to shed light on the issue. Due to the relatively small number of colitis-associated colorectal cancer-related cases, many aspects are not yet clear. As its incidence increases significantly, we should carry out large-scale, multicenter population-based cohort studies to formulate effective proactive preventive measures to reduce the occurrence of tumors; meanwhile, further studies on its cancerous mechanism will help to clarify the process of tumor development and progression, laying a theoretical basis for clinical early prevention as well as the selection of therapeutic targets.
-
3. Some other issues we need to be aware of:
Targeting molecules related to MDSCs can lead to anti-tumor effects that may be difficult to replicate in a clinical environment. Firstly, equivalence from mouse tumor models to human MDSC therapies is a challenge. Second, might there be functional differences between human and murine cell subpopulations? Human neutrophils may possess a more potent ability to kill tumor cells. Long-term inhibition of all neutrophils may be undesirable.
The activity of MDSCs may vary depending on the type of cancer and the stage of the disease. The types of MDSCs that play a major role may also be different in different cancer types. For example, PMN-MDSCs are the predominant cell population undergoing proliferation in various forms of cancer. However, in tumors like melanoma, M-MDSCs play a more critical role. Secondly, the mechanisms by which MDSCs may mediate inhibitory activity may be different at different stages of cancer. Therefore, it is important to identify the population of patients targeting MDSCs and their choice of drugs for different targets.
Moreover, different subtypes of MDSC such as PMN-MDSCs and M-MDSCs have different differentiation pathways, and different mechanisms of activity inhibition. Targeting a specific group of cells alone may not be effective in managing tumor growth. It is important to explore the combined targeting of multiple therapy approaches to effectively control tumor progression. There is a subset of patients who actually have no increase in MDSCs. Therefore, MDSC targeting may not be effective in these patients.
Therefore, the selection of therapeutic targets is important. And finding combinations of different drugs for different biological pathways may be a more effective way to target MDSCs. However, the toxicity potential of the drugs used, and the appropriate maximum clinical dose are critical issues to consider. Therefore, when selecting patients to be enrolled in the study, we need to analyze the tumor in detail, such as focusing on the detailed tumor stage and so on. At the same time, we should provide rational and individualized drug treatment plans for different categories of CAC patients. Moreover, we should focus on the development of preclinical models to achieve the transition from preclinical to clinical, and explore the reasonable combinations of treatments that can be adopted in the clinic.
Conclusion
Colitis-associated colorectal cancer is a specific form of colorectal cancer that develops as a result of inflammatory bowel disease (IBD). MDSCs, short for myeloid-derived suppressor cells, are a group of immature myeloid cells with immunosuppressive abilities that are crucial in the progression of inflammation and cancer. There is increasing evidence suggesting that MDSCs play a crucial role in the progression of colitis-associated colorectal cancer. Under pathological states such as inflammatory microenvironment and tumor, multiple immune pathways such as STAT3, NF-κB, COX-2/PGE2 activate to recruit MDSCs to exert the corresponding immunosuppressive functions, while some inflammatory cytokines and chemokines can promote the proliferation of MDSCs and stimulate MDSCs to release reactive oxygen radicals (ROS), inducible nitric oxide synthase (iNOS), Arginase 1 (Arg-1), and other compounds, these compounds impede T cell activity and induce T cell apoptosis, and can cause intestinal cell damage by changing epithelial permeability, destroying the intestinal epithelial barrier, and mismanaging the intestinal flora, leading to heterogeneous hyperplasia of intestinal epithelial cells and proliferation of tumor cells, as well as prompting intestinal epithelial cells to undergo irreversible cancerous changes, and transforming from inflammatory bowel disease to colorectal cancer.
Immunotherapy has opened new avenues for the treatment of some malignancies. However, those used at this stage, e.g., Monoclonal antibodies targeting PD-1/PD-L1, have good efficacy only in some patients. Therefore, improving the effectiveness of immunotherapy is an important research direction. W what’s more, this paper summarizes the strategies for targeting MDSC-related therapies as well as the possible strategies for treating CAC against MDSC at this stage, to provide a reference for the discovery of more potential targets for MDSC in the future. Although there are many therapeutic targets for MDSC, the key questions of how to better improve T-cell efficacy and how to accurately combat MDSC remain to be revealed in future clinical studies. Perhaps in the near future, MDSC targeted therapy may open a new era of tumor immunotherapy.
Data availability
No data was used for the research described in the article.
References
Akdeniz N, Kaplan MA, Uncu D, İnanç M, Kaya S, Dane F, Işıkdoğan A (2021) The comparison of FOLFOX regimens with different doses of 5-FU for the adjuvant treatment of colorectal cancer: a multicenter study. Int J Colorectal Dis 36(6):1311–1319. https://doi.org/10.1007/s00384-021-03888-9
André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Diaz LA Jr (2020) Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 383(23):2207–2218. https://doi.org/10.1056/NEJMoa2017699
André T, Falcone A, Shparyk Y, Moiseenko F, Polo-Marques E, Csöszi T, Saunders MP (2023) Trifluridine-tipiracil plus bevacizumab versus capecitabine plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer ineligible for intensive therapy (SOLSTICE): a randomised, open-label phase 3 study. Lancet Gastroenterol Hepatol 8(2):133–144. https://doi.org/10.1016/s2468-1253(22)00334-x
Bitsch R, Kurzay A, Özbay Kurt F, De La Torre C, Lasser S, Lepper A, Umansky V (2022) STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J Immunother Cancer 10(3):e004384. https://doi.org/10.1136/jitc-2021-004384
Boige V, Blons H, François E, Ben Abdelghani M, Phelip JM, Le Brun-Ly V, Bouché O (2023) Maintenance therapy with cetuximab after FOLFIRI plus cetuximab for ras wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Netw Open 6(9):e2333533. https://doi.org/10.1001/jamanetworkopen.2023.33533
Chen X, Qiu T, Zhu Y, Sun J, Li P, Wang B, Gu Y (2019) A single-arm, phase II study of apatinib in refractory metastatic colorectal cancer. Oncologist 24(7):883-e407. https://doi.org/10.1634/theoncologist.2019-0164
Chen H, Pan Y, Zhou Q, Liang C, Wong CC, Zhou Y, Yu J (2022a) METTL3 inhibits antitumor immunity by targeting m(6)A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology 163(4):891–907. https://doi.org/10.1053/j.gastro.2022.06.024
Chen Z, Zhang X, Xing Z, Lv S, Huang L, Liu J, He Y (2022b) OLFM4 deficiency delays the progression of colitis to colorectal cancer by abrogating PMN-MDSCs recruitment. Oncogene 41(22):3131–3150. https://doi.org/10.1038/s41388-022-02324-8
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34. https://doi.org/10.1016/s1470-2045(08)70285-7
Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Wong CC (2017) Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun 8(1):517. https://doi.org/10.1038/s41467-017-00530-7
Denda T, Sakai D, Hamaguchi T, Sugimoto N, Ura T, Yamazaki K, Yoshino T (2019) Phase II trial of aflibercept with FOLFIRI as a second-line treatment for Japanese patients with metastatic colorectal cancer. Cancer Sci 110(3):1032–1043. https://doi.org/10.1111/cas.13943
Ducarouge B, Redavid A-R, Victoor C, Chira R, Fonseca A, Hervieu M, Bernet A (2023) Netrin-1 blockade inhibits tumor associated Myeloid-derived suppressor cells, cancer stemness and alleviates resistance to chemotherapy and immune checkpoint inhibitor. Cell Death Differ 30(10):2201–2212. https://doi.org/10.1038/s41418-023-01209-x
Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, Paulson AS (2023a) Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine 58:101917. https://doi.org/10.1016/j.eclinm.2023.101917
Fakih M, Sandhu J, Lim D, Li X, Li S, Wang C (2023b) Regorafenib, ipilimumab, and nivolumab for patients with microsatellite stable colorectal cancer and disease progression with prior chemotherapy: a phase 1 nonrandomized clinical trial. JAMA Oncol 9(5):627–634. https://doi.org/10.1001/jamaoncol.2022.7845
Friis S, Riis AH, Erichsen R, Baron JA, Sørensen HT (2015) Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk. Ann Intern Med 163(5):347–355. https://doi.org/10.7326/M15-0039
Gabrilovich DI (2017) Myeloid-derived suppressor cells. Cancer Immunol Res 5(1):3–8. https://doi.org/10.1158/2326-6066.Cir-16-0297
Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G (2020) Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 17(12):725–741. https://doi.org/10.1038/s41571-020-0413-z
Garcia-Carbonero R, Rivera F, Maurel J, Ayoub JP, Moore MJ, Cervantes A, Tabernero J (2014) An open-label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy for metastatic colorectal cancer. Oncologist 19(4):350–351. https://doi.org/10.1634/theoncologist.2014-0028
Grivennikov S, Karin E, Terzic J, Mucida D, Yu G, Vallabhapurapu S, Karin MJC (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15(2):103–113. https://doi.org/10.1016/j.ccr.2009.01.001
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Laurent D (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312. https://doi.org/10.1016/s0140-6736(12)61900-x
Gürlevik E, Fleischmann-Mundt B, Brooks J, Demir I, Steiger K, Ribback S, Kühnel FJG (2016) Administration of gemcitabine after pancreatic tumor resection in mice induces an antitumor immune response mediated by natural killer. Cells 151(2):338-350.e337. https://doi.org/10.1053/j.gastro.2016.05.004
Haag GM, Springfeld C, Grün B, Apostolidis L, Zschäbitz S, Dietrich M, Jaeger D (2022) Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer - The PICCASSO phase I trial. Eur J Cancer 167:112–122. https://doi.org/10.1016/j.ejca.2022.03.017
Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel S-E, Daher M, Diab A (2022) Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 40(5):509-523.e506. https://doi.org/10.1016/j.ccell.2022.04.004
Hashimoto A, Fukumoto T, Zhang R, Gabrilovich D (2020) Selective targeting of different populations of myeloid-derived suppressor cells by histone deacetylase inhibitors. Cancer Immunol Immunother 69(9):1929–1936. https://doi.org/10.1007/s00262-020-02588-7
Hayter CR, Huff-Winters C, Paszat L, Youssef YM, Shelley WE, Schulze K (2000) A prospective trial of short-course radiotherapy plus chemotherapy for palliation of dysphagia from advanced esophageal cancer. Radiother Oncol 56(3):329–333. https://doi.org/10.1016/s0167-8140(00)00225-5
Hegde S, Leader AM, Merad M (2021) MDSC: Markers, development, states, and unaddressed complexity. Immunity 54(5):875–884. https://doi.org/10.1016/j.immuni.2021.04.004
Heinrich MC, Jones RL, George S, Gelderblom H, Schöffski P, von Mehren M, Bauer S (2024) Ripretinib versus sunitinib in gastrointestinal stromal tumor: ctDNA biomarker analysis of the phase 3 INTRIGUE trial. Nat Med 30(2):498–506. https://doi.org/10.1038/s41591-023-02734-5
Hengesbach LM, Hoag KA (2004) Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J Nutr 134(10):2653–2659. https://doi.org/10.1093/jn/134.10.2653
Hsu N-Y, Nayar S, Gettler K, Talware S, Giri M, Alter I, Cho JH (2022) NOX1 is essential for TNFα-induced intestinal epithelial ROS secretion and inhibits M cell signatures. Gut 72(4):654–662. https://doi.org/10.1136/gutjnl-2021-326305
Hu S, Fang Y, Chen X, Cheng T, Zhao M, Du M, Chen ZJ (2021) cGAS restricts colon cancer development by protecting intestinal barrier integrity. Proc Natl Acad Sci 118(23):e2105747118. https://doi.org/10.1073/pnas.2105747118
Huang T, Cheng X, Chahoud J, Sarhan A, Tamboli P, Rao P, Lu X (2020) Effective combinatorial immunotherapy for penile squamous cell carcinoma. Nat Commun. https://doi.org/10.1038/s41467-020-15980-9
Ibrahim ML, Klement JD, Lu C, Redd PS, Xiao W, Yang D, Liu K (2018) Myeloid-derived suppressor cells produce IL-10 to elicit DNMT3b-dependent IRF8 silencing to promote colitis-associated colon tumorigenesis. Cell Rep 25(11):3036-3046.e3036. https://doi.org/10.1016/j.celrep.2018.11.050
Jiang TY, Cui XW, Zeng TM, Pan YF, Lin YK, Feng XF, Wang HY (2023) PTEN deficiency facilitates gemcitabine efficacy in cancer by modulating the phosphorylation of PP2Ac and DCK. Sci Transl Med 15(704):eadd7464. https://doi.org/10.1126/scitranslmed.add7464
Johnson M, Dudek AZ, Sukari A, Call J, Kunk PR, Lewis K, Goldman JW (2022) ARRY-382 in Combination with pembrolizumab in patients with advanced solid tumors: results from a phase 1b/2 Study. Clin Cancer Res 28(12):2517–2526. https://doi.org/10.1158/1078-0432.Ccr-21-3009
Kang YK, Ryu MH, Park SH, Kim JG, Kim JW, Cho SH, Oh SC (2018) Efficacy and safety findings from DREAM: a phase III study of DHP107 (oral paclitaxel) versus i.v. paclitaxel in patients with advanced gastric cancer after failure of first-line chemotherapy. Ann Oncol 29(5):1220–1226. https://doi.org/10.1093/annonc/mdy055
Karpisheh V, Nikkhoo A, Hojjat-Farsangi M, Namdar A, Azizi G, Ghalamfarsa G, Jadidi-Niaragh F (2019) Prostaglandin E2 as a potent therapeutic target for treatment of colon cancer. Prostaglandins Other Lipid Mediat 144:106338. https://doi.org/10.1016/j.prostaglandins.2019.106338
Ke Z, Wang C, Wu T, Wang W, Yang Y, Dai YJC (2020) PAR2 deficiency enhances myeloid cell-mediated immunosuppression and promotes colitis-associated tumorigenesis. Cancer Lett 469:437–446. https://doi.org/10.1016/j.canlet.2019.11.015
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Vogel A (2023) Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401(10391):1853–1865. https://doi.org/10.1016/s0140-6736(23)00727-4
Kim W, Chu TH, Nienhüser H, Jiang Z, Del Portillo A, Remotti HE, Wang TC (2021) PD-1 signaling promotes tumor-infiltrating myeloid-derived suppressor cells and gastric tumorigenesis in mice. Gastroenterology 160(3):781–796. https://doi.org/10.1053/j.gastro.2020.10.036
Kim R, Hashimoto A, Markosyan N, Tyurin V, Tyurina Y, Kar G, Gabrilovich DJN (2022) Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 612(7939):338–346. https://doi.org/10.1038/s41586-022-05443-0
Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, Saltz L (2015) Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol 33(34):4032–4038. https://doi.org/10.1200/jco.2015.63.2497
Krishnamoorthy M, Gerhardt L, Maleki Vareki S (2021) Immunosuppressive effects of myeloid-derived suppressor cells in cancer and immunotherapy. Cells 10(5):1170. https://doi.org/10.3390/cells10051170
Laubach JP, Liu C-J, Raje NS, Yee AJ, Armand P, Schlossman RL, Ghobrial IM (2019) A phase I/II study of evofosfamide, a hypoxia-activated prodrug with or without bortezomib in subjects with relapsed/refractory multiple myeloma. Clin Cancer Res 25(2):478–486. https://doi.org/10.1158/1078-0432.CCR-18-1325
Laws M, Walker E, Cozzi F, Ampie L, Jung M, Burton E, Brown DJF (2023) Glioblastoma may evade immune surveillance through primary cilia-dependent signaling in an IL-6 dependent manner. Front Oncol 13:1279923. https://doi.org/10.3389/fonc.2023.1279923
Lee K, Kim HM (2011) A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor. Arch Pharmacal Res 34(10):1583–1585. https://doi.org/10.1007/s12272-011-1021-3
Li T, Li X, Zamani A, Wang W, Lee C, Li M, Chen YJN (2020) c-Rel Is a myeloid checkpoint for cancer immunotherapy. Nat Cancer 1(5):507–517. https://doi.org/10.1038/s43018-020-0061-3
Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, DePinho RA (2019) KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 35(4):559-572.e557. https://doi.org/10.1016/j.ccell.2019.02.008
Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, Chevriaux A, Ghiringhelli F (2016a) Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res 76(18):5241–5252. https://doi.org/10.1158/0008-5472.Can-15-3164
Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, Chevriaux A, Ghiringhelli F (2016b) Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX–bevacizumab drug treatment regimen. Can Res 76(18):5241–5252. https://doi.org/10.1158/0008-5472.CAN-15-3164
Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, Wang X (2020) TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett 469:173–185. https://doi.org/10.1016/j.canlet.2019.10.020
Liu H, Lou J, Liu Y, Liu Z, Xie J, Sun J, Han W (2022) Intestinal epithelial cell autophagy deficiency suppresses inflammation-associated colon tumorigenesis. Molecular Thera Py—Nucleic Acids 28:35–46. https://doi.org/10.1016/j.omtn.2022.02.012
Liu H, Deng R, Zhu C-W, Han H-K, Zong G-F, Ren L, Lu Y (2023a) Rosmarinic acid in combination with ginsenoside Rg1 suppresses colon cancer metastasis via co-inhition of COX-2 and PD1/PD-L1 signaling axis. Acta Pharmacol Sin 45(1):193–208. https://doi.org/10.1038/s41401-023-01158-8
Liu X, Tang R, Xu J, Tan Z, Liang C, Meng Q, Shi S (2023b) CRIP1 fosters MDSC trafficking and resets tumour microenvironment via facilitating NF-κB/p65 nuclear translocation in pancreatic ductal adenocarcinoma. Gut 72(12):2329–2343. https://doi.org/10.1136/gutjnl-2022-329349
Luke JJ, Fakih M, Schneider C, Chiorean EG, Bendell J, Kristeleit R, Naing A (2023) Phase I/II sequencing study of azacitidine, epacadostat, and pembrolizumab in advanced solid tumors. Br J Cancer 128(12):2227–2235. https://doi.org/10.1038/s41416-023-02267-1
Ma Z, Zhen Y, Hu C, Yi HJF (2020) Myeloid-derived suppressor cell-derived arginase-1 oppositely modulates IL-17A and IL-17F Through the ESR/STAT3 pathway during colitis in mice. Front Immunol 11:687. https://doi.org/10.3389/fimmu.2020.00687
Mabrouk AA, El-Mezayen NS, Tadros MI, El-Gazayerly ON, El-Refaie WM (2023) Novel mucoadhesive celecoxib-loaded cubosomal sponges: anticancer potential and regulation of myeloid-derived suppressor cells in oral squamous cell carcinoma. Eur J Pharm Biopharm 182:62–80. https://doi.org/10.1016/j.ejpb.2022.12.003
Mao Y, Eissler N, Blanc KL, Johnsen JI, Kogner P, Kiessling R (2016) Targeting suppressive myeloid cells potentiates checkpoint inhibitors to control spontaneous neuroblastoma. Clin Cancer Res 22(15):3849–3859. https://doi.org/10.1158/1078-0432.CCR-15-1912
Mehta AR, Armstrong AJ (2016) Tasquinimod in the treatment of castrate-resistant prostate cancer - current status and future prospects. Ther Adv Urol 8(1):9–18. https://doi.org/10.1177/1756287215603558
Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Hainsworth J (2019) Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 20(4):518–530. https://doi.org/10.1016/s1470-2045(18)30904-5
Michaelis KA, Norgard MA, Zhu X, Levasseur PR, Sivagnanam S, Liudahl SM, Marks DL (2019) The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat Commun 10(1):4682. https://doi.org/10.1038/s41467-019-12657-w
Minematsu H, Afify S, Sugihara Y, Hassan G, Zahra M, Seno A, Seno M (2022) Cancer stem cells induced by chronic stimulation with prostaglandin E2 exhibited constitutively activated PI3K axis. Sci Rep 12(1):15628. https://doi.org/10.1038/s41598-022-19265-7
Mo W, Liu S, Zhao X, Wei F, Li Y, Sheng X, Guo H (2023) ROS Scavenging Nanozyme Modulates Immunosuppression for Sensitized Cancer Immunotherapy. Adv Healthc Mater 12(21):e2300191. https://doi.org/10.1002/adhm.202300191
Morano F, Corallo S, Lonardi S, Raimondi A, Cremolini C, Rimassa L, Pietrantonio F (2019) Negative hyperselection of patients with ras and braf wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol 37(33):3099–3110. https://doi.org/10.1200/jco.19.01254
Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag C, Laversanne M, Bray FJG (2023) Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72(2):338–344. https://doi.org/10.1136/gutjnl-2022-327736
Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Pasparakis M (2007) Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446(7135):557–561. https://doi.org/10.1038/nature05698
Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Linehan DC (2016) Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 17(5):651–662. https://doi.org/10.1016/s1470-2045(16)00078-4
Olson DJ, Luke JJ (2023) Myeloid maturity: ATRA to enhance anti-PD-1? Clin Cancer Res 29(7):1167–1169. https://doi.org/10.1158/1078-0432.Ccr-22-3652
Omahony C, Clooney A, Clarke S, Aguilera M, Gavin A, Simnica D, Melgar S (2023) Dietary-induced bacterial metabolites reduce inflammation and inflammation associated cancer via vitamin D pathway. Int J Mol Sci 24(3):1864. https://doi.org/10.3390/ijms24031864
Qin S, Li J, Bai Y, Deng Y, Yang L, Xu RH, Shen L (2021) Quality-adjusted survival in patients with metastatic colorectal cancer treated with fruquintinib in the FRESCO trial. Future Oncol 17(15):1923–1931. https://doi.org/10.2217/fon-2020-1215
Qin G, Liu S, Liu J, Hu H, Yang L, Zhao Q (2023) Overcoming resistance to immunotherapy by targeting GPR84 in myeloid-derived suppressor cells. Signal Transduct Target Therap 8(1):164. https://doi.org/10.1038/s41392-023-01388-6
Reilley MJ, McCoon P, Cook C, Lyne P, Kurzrock R, Kim Y, Hong DS (2018) STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J Immunother Cancer 6(1):119. https://doi.org/10.1186/s40425-018-0436-5
Ruiz-Saenz A, Atreya CE, Wang C, Pan B, Dreyer CA, Brunen D, Coppé J-P (2023) A reversible SRC-relayed COX2 inflammatory program drives resistance to BRAF and EGFR inhibition in BRAFV600E colorectal tumors. Nature Cancer 4(2):240–256. https://doi.org/10.1038/s43018-022-00508-5
Saeed A, Park R, Pathak H, Al-Bzour AN, Dai J, Phadnis M, Saeed A (2024) Clinical and biomarker results from a phase II trial of combined cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC cohort. Nat Commun 15(1):1533. https://doi.org/10.1038/s41467-024-45960-2
Sarkar O, Donninger H, Al Rayyan N, Chew L, Stamp B, Zhang X, Yaddanapudi K (2023) Monocytic MDSCs exhibit superior immune suppression via adenosine and depletion of adenosine improves efficacy of immunotherapy. Sci Adv 9(26):3736. https://doi.org/10.1126/sciadv.adg3736
Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Ferrara N (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 25(8):911–920. https://doi.org/10.1038/nbt1323
Siegel R, Wagle N, Cercek A, Smith R, Jemal A (2023) Colorectal cancer statistics, 2023. CA Cancer J Clin 73(3):233–254. https://doi.org/10.3322/caac.21772
Song Q, Zhao H, Zheng C, Wang K, Gao H, Feng Q, Wang L (2021) A bioinspired versatile spore coat nanomaterial for oral probiotics delivery. Adv Func Mater 31(41):2104994. https://doi.org/10.1002/adfm.202104994
Spaander M, Zauber A, Syngal S, Blaser M, Sung J, You Y, Kuipers EJ (2023) Young-onset colorectal cancer. Nat Rev Dis Primers 9(1):21. https://doi.org/10.1038/s41572-023-00432-7
Strickler JH, Cercek A, Siena S, André T, Ng K, Van Cutsem E, Shao S (2023) Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol 24(5):496–508. https://doi.org/10.1016/S1470-2045(23)00150-X
Thiele Orberg E, Fan H, Tam AJ, Dejea CM, Destefano Shields CE, Wu S, Housseau F (2017) The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol 10(2):421–433. https://doi.org/10.1038/mi.2016.53
Tobin RP, Cogswell DT, Cates VM, Davis DM, Borgers JSW, Van Gulick RJ, McCarter MD (2023) Targeting MDSC differentiation using ATRA: a phase I/II clinical trial combining pembrolizumab and all-trans retinoic acid for metastatic melanoma. Clin Cancer Res 29(7):1209–1219. https://doi.org/10.1158/1078-0432.Ccr-22-2495
Tsien CI, Pugh SL, Dicker AP, Raizer JJ, Matuszak MM, Lallana EC, Mehta MP (2023) NRG Oncology/RTOG1205: a randomized phase ii trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J Clin Oncol 41(6):1285–1295. https://doi.org/10.1200/jco.22.00164
Tsukamoto H, Nishikata R, Senju S, Nishimura Y (2013) Myeloid-derived suppressor cells attenuate th1 development through il-6 production to promote tumor progression. Cancer Immunol Res 1(1):64–76. https://doi.org/10.1158/2326-6066.CIR-13-0030
Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Wang TC (2008) Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14(5):408–419. https://doi.org/10.1016/j.ccr.2008.10.011
Vallilas C, Sarantis P, Kyriazoglou A, Koustas E, Theocharis S, Papavassiliou AG, Karamouzis MV (2021) Gastrointestinal Stromal Tumors (GISTs): Novel Therapeutic Strategies with Immunotherapy and Small Molecules. Int J Mol Sci 22(2):493. https://doi.org/10.3390/ijms22020493
Wan D, Yang Y, Liu Y, Cun X, Li M, Xu S, He Q (2020) Sequential depletion of myeloid-derived suppressor cells and tumor cells with a dual-pH-sensitive conjugated micelle system for cancer chemoimmunotherapy. J Control Release 317:43–56. https://doi.org/10.1016/j.jconrel.2019.11.011
Wang Y, Ding Y, Deng Y, Zheng Y, Wang S (2020) Role of myeloid-derived suppressor cells in the promotion and immunotherapy of colitis-associated cancer. J Immunother Cancer 8(2):e000609. https://doi.org/10.1136/jitc-2020-000609
Wang C, Zheng X, Zhang J, Jiang X, Wang J, Li Y, Luo M (2023a) CD300ld on neutrophils is required for tumour-driven immune suppression. Nature 621(7980):830–839. https://doi.org/10.1038/s41586-023-06511-9
Wang L, Mou H, Hou X, Liao Q (2023b) Case report: a case of complete clinical response in a patient experiencing high microsatellite instability unresectable colon cancer being treated with a PD-L1 inhibitor after interstitial pneumonia. Front Oncol 13:1126769. https://doi.org/10.3389/fonc.2023.1126769
Wang Q, Long G, Luo H, Zhu X, Han Y, Shang Y (2023c) S100A8/A9: An emerging player in sepsis and sepsis-induced organ injury. Biomed Pharmacother 168:115674. https://doi.org/10.1016/j.biopha.2023.115674
Wang X, Zhang D, Yang Y, Li X, Li H, Zhang X, Ma XJ (2023d) viaSuppression of microRNA-222-3p ameliorates ulcerative colitis and colitis-associated colorectal cancer to protect against oxidative stress targeting BRG1 to activate Nrf2/HO-1 signaling pathway. Front Immunol 14:1089809. https://doi.org/10.3389/fimmu.2023.1089809
Wang Y, Liu H, Zhang Z, Bian D, Shao K, Wang S, Ding YJJ (2023e) G-MDSC-derived exosomes mediate the differentiation of M-MDSC into M2 macrophages promoting colitistocancer transition. J Immunother Cancer 11(6):e006166. https://doi.org/10.1136/jitc-2022-006166
Webster-Clark M, Keil AP, Robert N, Frytak JR, Boyd M, Stürmer T, Lund JL (2022) Comparing trial and real-world adjuvant oxaliplatin delivery in patients with stage III colon cancer using a longitudinal cumulative dose. JAMA Oncol 8(12):1821–1824. https://doi.org/10.1001/jamaoncol.2022.4445
Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, Serafini P (2015) Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 21(1):39–48. https://doi.org/10.1158/1078-0432.Ccr-14-1711
Wu L, Xu Y, Zhao H, Zhou Y, Chen Y, Yang S, Li YJT (2022a) FcγRIIB potentiates differentiation of myeloid-derived suppressor cells to mediate tumor immunoescape. Theranostics 12(2):842–858. https://doi.org/10.7150/thno.66575
Wu Y, Yi M, Niu M, Mei Q, Wu K (2022b) Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer 21(1):184. https://doi.org/10.1186/s12943-022-01657-y
Xie Z, Ikegami T, Ago Y, Okada N, Tachibana M (2020) Valproic acid attenuates CCR2-dependent tumor infiltration of monocytic myeloid-derived suppressor cells, limiting tumor progression. OncoImmunology 9(1):1734268. https://doi.org/10.1080/2162402X.2020.1734268
Xu RH, Shen L, Wang KM, Wu G, Shi CM, Ding KF, Yu H (2017) Famitinib versus placebo in the treatment of refractory metastatic colorectal cancer: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin J Cancer 36(1):97. https://doi.org/10.1186/s40880-017-0263-y
Xun J, Zhou S, Lv Z, Wang B, Luo H, Zhang L, Zhang Q (2023) Dioscin modulates macrophages polarization and MDSCs differentiation to inhibit tumorigenesis of colitis-associated colorectal cancer. Int Immunopharmacol 117:109839. https://doi.org/10.1016/j.intimp.2023.109839
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Moses HL (2008) Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13(1):23–35. https://doi.org/10.1016/j.ccr.2007.12.004
Yang Y, Gharaibeh RZ, Newsome RC, Jobin C (2020) Amending microbiota by targeting intestinal inflammation with TNF blockade attenuates development of colorectal cancer. Nature Cancer 1(7):723–734. https://doi.org/10.1038/s43018-020-0078-7
Yang X, Li G, Lou P, Zhang M, Yao K, Xiao J, Lv C (2024) Excessive nucleic acid R-loops induce mitochondria-dependent epithelial cell necroptosis and drive spontaneous intestinal inflammation. Proc Natl Acad Sci U S A 121(1):e2307395120. https://doi.org/10.1073/pnas.2307395120
Yap TA, Vieito M, Baldini C, Sepúlveda-Sánchez JM, Kondo S, Simonelli M, Melisi D (2021) First-in-human phase I study of a next-generation, oral, TGFβ receptor 1 inhibitor, LY3200882, in patients with advanced cancer. Clin Cancer Res 27(24):6666–6676. https://doi.org/10.1158/1078-0432.Ccr-21-1504
Ye Y, Wang X, Jeschke U, von Schönfeldt V (2020) COX-2-PGE(2)-EPs in gynecological cancers. Arch Gynecol Obstet 301(6):1365–1375. https://doi.org/10.1007/s00404-020-05559-6
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181(8):5791–5802. https://doi.org/10.4049/jimmunol.181.8.5791
Yvellez OV, Rai V, Sossenheimer PH, Hart J, Turner JR, Weber C, Rubin DT (2021) Cumulative histologic inflammation predicts colorectal neoplasia in ulcerative colitis: a validation study. Inflamm Bowel Dis 27(2):203–206. https://doi.org/10.1093/ibd/izaa047
Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, Greten TF (2020) Gut microbiome directs hepatocytes to recruit MDSCS and promote cholangiocarcinoma. Cancer Discov 11(5):1248–1267. https://doi.org/10.1158/2159-8290.CD-20-0304
Zhang J, Simpson CM, Berner J, Chong HB, Fang J, Ordulu Z, Bar-Peled L (2023) Systematic identification of anticancer drug targets reveals a nucleus-to-mitochondria ROS-sensing pathway. Cell 186(11):2361-2379.e2325. https://doi.org/10.1016/j.cell.2023.04.026
Zhang K, Zakeri A, Alban T, Dong J, Ta HM, Zalavadia AH, Wang LL (2024) VISTA promotes the metabolism and differentiation of myeloid-derived suppressor cells by STAT3 and polyamine-dependent mechanisms. Cell Rep 43(1):113661. https://doi.org/10.1016/j.celrep.2023.113661
Zhou AP, Bai Y, Song Y, Luo H, Ren XB, Wang X, Ma J (2019) Anlotinib versus sunitinib as first-line treatment for metastatic renal cell carcinoma: a randomized phase II clinical trial. Oncologist 24(8):e702–e708. https://doi.org/10.1634/theoncologist.2018-0839
Zhou Q, Peng Y, Ji F, Chen H, Kang W, Chan LS, Wong CC (2023) Targeting of SLC25A22 boosts the immunotherapeutic response in KRAS-mutant colorectal cancer. Nat Commun 14(1):4677. https://doi.org/10.1038/s41467-023-39571-6
Zhu W, Chu H, Zhang Y, Luo T, Yu H, Zhu H, Yang WJC (2023) Fructose-1,6-bisphosphatase 1 dephosphorylates IκBα and suppresses colorectal tumorigenesis. Cell Res 33(3):245–257. https://doi.org/10.1038/s41422-022-00773-0
Funding
The current research was funded by the Research Project of the Jiangsu Commission of Health (Grant No.K2023062), and National Natural Science Foundation of China (82370533).
Author information
Authors and Affiliations
Contributions
Conceptualization was done by Kai Yin and Kang Wang. Kang Wang was responsible for writing the original draft. Kai Yin, Kang Wang, and Yun Wang were involved in reviewing, discussing, and supervising this review. All authors have reviewed and endorsed the final draft of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, K., Wang, Y. & Yin, K. Role played by MDSC in colitis-associated colorectal cancer and potential therapeutic strategies. J Cancer Res Clin Oncol 150, 243 (2024). https://doi.org/10.1007/s00432-024-05755-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05755-w