Abstract
Background
Small round cell tumor (SRCT) is a group of malignancy with similar optical microscopic morphology. Despite its low incidence, SRCT has a high malignant degree and poor prognosis. Besides, atypical clinical symptoms make it difficult in preoperative diagnosis.
Case report
A 67-year-old man was presented to the outpatient service with dysuria and weak urine stream lasting for 3 months. After oral treatment with tamsulosin and finasteride for 2 months, the symptoms worsen. Transurethral prostate holmium laser enucleation was operated and postoperative pathology result revealed small blue round cell malignant tumor. Further immunohistochemistry and fluorescence in situ hybridization examination indicated Ewing-like SRCT. So a Da Vinci Robotic prostatectomy was performed further and whole-genome sequencing was conducted. Several gene mutations including RAF1, ARID1A, SMARCA4, and BCL2L11 were found but no FDA-approved drug could treat specifically. Then the patient received Ewing-type therapeutic regimens treatment and has been followed up to date (over 24 months).
Conclusion
Because of its non-elevated serum PSA level, prostate SRCT is often ignored as a possibility of malignant tumor and regarded as benign prostatic hyperplasia (BPH). The possibility of prostate SRCT need to be considered if dysuria symptoms could not alleviate significantly after a period of oral treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small round cell tumor (SRCT) is a heterogeneous tumor composed of relatively small undifferentiated cells that are round or oval in shape and densely arranged. It has less cytoplasm, round nuclei, uniform distribution, rough chromatin, and small or inconspicuous nucleoli under light microscopy (Rajwanshi et al. 2009). Despite having similar cell morphology under microscope, the pathological entities of SRCT may come from extremely different lineages, including (1) epithelial tumors, such as small cell carcinoma; (2) mesenchymal tumors, including malignant solid tumors in children and other small circles cell sarcoma; (3) tumors with overlapping features such as lymphoma and melanoma. We now report a rare Ewing-like small round cell tumor that occurs in the prostate with unique genetic phenotype.
Case presentation
A 67-year-old man was presented to his local hospital outpatient service with dysuria and weak urine stream for 3 months. Prostate ultrasound indicated benign prostatic hyperplasia (BPH) and no obvious nodule was detected. Besides, the level of serum prostate-specific antigen (PSA) was not elevated. So he was given oral treatment with tamsulosin and finasteride for 2 months, but he felt the dysuria symptoms gradually worsened.
To further deal with dysuria, the patient was hospitalized in local hospital. Physical examination upon admission of the patient showed the prostate had increased volume with hard texture and the central groove disappeared, oppressed the rectum. No other apparently positive signs were found. He had no special medical, family, and psycho-social history except chronic B-viral hepatitis for over 30 years and denied any alcohol, drug or smoke consumption. So he underwent transurethral Holmium laser prostate surgery. Postoperative pathology result revealed small blue round cell malignant tumor (Fig. 1). To further clarify the pathological types of tumor tissue, immunohistochemistry (IHC) was performed, suggesting high-grade prostate cancer with neuroendocrine and neuroectodermal differentiation (Fig. 2). To further confirm the diagnosis, fluorescence in situ hybridization (FISH) examination was performed and found no SYT gene disruption and rearrangement, and no EWSR1/FLI1 fusion gene.
Postoperative pathology result of this case. A Small round blue cells with diffuse growth pattern, B bundles in part, C, D areas of neoplastic necrosis (the black arrow shows), E pale tumor cell nuclei with round, ovoid, and short spindle shape, delicate chromatin with visible nucleoli, F areas of pathological mitotic figures (The black arrow shows). Bar: 100X, 100 μm; 200X, 50 μm; 400X, 20 μm
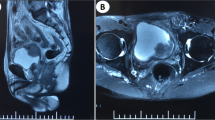
The dysuria symptoms significantly relieved but aggravated again 1 month after operation, and the symptoms of dribbling at the end of urination appeared, accompanied with increased frequency of urination at night (1-h interval). For further treatment, the patient presented to our hospital. The patient underwent magnetic resonance (MR) examination and found prostate enlargement with irregular masses, unclear boundary between central lobe and peripheral lobe, uneven signal on T2WI image, obviously high signal on DWI image (Fig. 3A–D). Further PET–CT found that abnormal glucose metabolism of the prostate was increased and the posterior wall of the bladder was involved but no distant organ metastasis was found (Fig. 3E). Therefore, after multiple disciplinary team discussion, a da Vinci robotic prostatectomy was performed. Tumor resection specimens were subjected to further whole-genome sequencing. The results suggested that there were three somatic variations that may have clinical significance, including RAF1 (CCDC6-RAF1 fusion), ARID1A, and SMARCA4. Moreover, one germline variation that may have clinical significance is BCL2L11 (2903-bp deletion). Besides, it was found that the tumor mutation burden was 2.33 and microsatellite stable (MSS). What is more, there were no mutation on ALK, BRAF, BRCA1/2, PD-L1, EGFR, EGFR2/3, HER2, KIT, KRAS, MET, NRAS, NTRK1/2/3, PDGFRA, PIK3CA, RET, ROS1, which have potential Food and Drug Administration (FDA)-approved targeted drugs for choosing. The test results did not find specific targeted drugs that could be used clinically in this patient at present.
Postoperative recovery was uneventful and the patient was discharged on the 11th postoperative day with smooth urination. Then the patient received four cycles of Ewing-type therapeutic regimens treatment (Vinorelbine Tartrate 30 mg on Day 1 + Epirubicin Hydrochloride 70 mg on Day 1–2 + cyclophosphamide 1 g on Day 1) every 3 weeks. He has been followed up to date and is currently undergoing stable follow-up for over 24 months.
Discussion
SRCT is a group of different histologic-type neoplasms including Ewing’s sarcoma (EWS)/primitive neuroectodermal tumors (PNET), Askin’s tumor, melanocytic neuroectodermal tumor, neuroblastoma, olfactory neuroblastoma, poorly differentiated synovial sarcoma, mesenchymal chondrosarcoma, rhabdomyosarcoma, Wilms’ tumor, desmoplastic small round cells tumor and so on. In addition, tumors with small cell morphology similar to SRCT contain non-Hodgkin’s lymphoma, round cell liposarcoma, extraskeletal myxoid chondrosarcoma, poorly differentiated malignant peripheral nerve sheath tumor, malignant melanoma, rhabdoid tumor, germ cell tumors, small cell carcinoma, Merkel cell carcinoma (Meis-Kindblom et al. 1996).
In this paper, we described a rare case of prostate SRCT due to dysuria which is never reported before. Its clinical manifestations lack typicality, and ultrasound, CT, MRI with other imaging examinations are not specific. Differential diagnosis is difficult with prostate cancer and BPH, which may cause treatment delay. Pathological examination is the main diagnostic basis.
SRCT has the same morphology and size, and they are diffusely distributed in tissues with poor differentiation, high malignancy, and poor prognosis. Further histologic type is defined by IHC (Sharma et al. 2017). Besides, genetic testing is another diagnostic tool that helps SRCT be correctly classified. Most classical SRCT cytogenetic features are chromosomal aberrations, mainly translocations and proliferation of chromosome fragments (Rabbitts 1994). Studies have suggested that chromosomal translocations are primary. Soft tissue tumor-specific chromosomal aberrations lead to the recombination and fusion of protein-coding genes, which can lead to the expression of cancer-promoting proteins. The combination of the two protein-coding genes facilitates fusion gene formation, which is translated into a fusion (chimeric) protein after transcription. These two mechanisms will lead to malignant transformation of tumors or uncontrolled cell proliferation (Xia and Barr 1990). In this case, the tumor type could not be classified into current classification, but showed EWS/ PNET like.
Ewing-like SRCT comprises tumor with EWSR1 rearrangements with a non-ETS family partner and tumor with non-EWSR1-ETS rearrangements (Pappo and Dirksen 2018; Antonescu 2014). The most frequent translocations in non-EWSR1-ETS rearranged SRCT occur between the CIC gene on chromosome 19 and DUX4 on chromosomes 4 and 10, resulting in t(4;19)(q35;q13) or t(10;19)(q26;q13) (Machado et al. 2016). In addition, about 4% of non-EWSR1-ETS rearranged SRCT has BCOR rearrangements or BCOR-CCNB3 fusions (Pierron et al. 2012). Besides, there have been tumor types with NFATC2 and PATZ1 fusion. Despite significant morphological overlap, most of these subsets tend to exhibit morphological features predicting potential molecular changes. Ewing’s sarcoma is the prototype of round cell sarcoma, while in CIC sarcoma, focal polymorphism and epithelial morphology may dominate. BCOR sarcomas usually exhibit spindle-like tumor cell population. NFATC2 sarcoma may show significant epithelioid features, while PATZ1 sarcoma usually has a sclerotic background (Sbaraglia et al. 2020). The CHIP and whole-genome sequencing result in our study showed non- EWSR1-ETS rearrangements, non-EWSR1-ETS rearrangements and non-BCOR-CCNB3 fusions, indicating a new found unique subtype.
There is currently no standard treatment for SRCT. Most treatments are performed with early surgical resection and are supplemented with radiotherapy and chemotherapy. Although it has a unique genetic mutation that is different from typical Ewing sarcoma or other well-defined Ewing-like SRCT, Ewing-type therapeutic regimens was chose as a follow-up treatment for this patient (Marino-Enriquez and Fletcher 2014). According to the whole-genome sequencing results, three somatic variations may have clinical significance, including RAF1 (CCDC6-RAF1 fusion), ARID1A, SMARCA4, and one germline variation BCL2L11. Cell experiments have shown that spontaneous activation of RAF1 can induce estrogen-independent growth of breast cancer cells and overexpression of continuously activated RAF1 protein can cause cell resistance to adriamycin and paclitaxel (El-Ashry et al. 1997; Davis et al. 2003). In addition, in vitro cell experiments showed that after the ARID1A gene was deleted, the ability of PARP inhibitors (Veliparib, Olaparib, Rucaparib, BMN673) to promote apoptosis was enhanced (Shen et al. 2015). Low ARID1A expression is associated with activation of the PI3K/mTOR signaling pathway (Huang et al. 2014). In vitro cell experiments have shown that ARID1A-deficient breast cancer cells have increased sensitivity to PI3K inhibitors (Buparlisib) and AKT inhibitors (MK-2206 and Perifosine) (Samartzis et al. 2014). In vitro cell experiments showed that ARID1A knockout lung cancer cells were sensitive to ionizing radiation, cisplatin, and UV (Watanabe et al. 2014). As for SMARCA4, in non-small cell lung cancer, SMARCA4 deletion is sensitive to combination of etoposide and DZNep (EZH2 inhibitor) (Fillmore et al. 2015). Besides, studies have confirmed that BCL2L11 deletion is associated with poor response to EGFR TKI treatment (Huang et al. 2015; Nie et al. 2015). Blocking these genes seems to be an effective treatment strategy but there are currently no drugs that specifically target. Furthermore, this case showed low tumor mutational load and MSS predicting poor immunotherapy responsiveness including PD-1/PD-L1 treatment (Rosenberg et al. 2016; Snyder et al. 2014; Rizvi et al. 2015; Le et al. 2015).
Although rare, this case highlights the need to consider malignant disease such as SRCT as a differential diagnosis for dysuria patient without elevated serum PSA level. Early detection and pathological examination is needed when there have unexpected disease progression.
Conclusion
Prostate Ewing-like SRCT is rare and difficult to preoperatively diagnose. Even through clinical features, imageology examination, frozen pathological examination, we cannot obtain an exact diagnosis. To find unexpected prostate SRCT, any suspicious details of prostate-related symptoms or clinical diagnosed BPH should be taken into account during the follow-up of drug treatment. More importantly, new targeted drugs for the disease need to be developed for clinical application in the future.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Antonescu C (2014) Round cell sarcomas beyond Ewing: emerging entities. Histopathology 64(1):26–37
Davis JM, Navolanic PM, Weinstein-Oppenheimer CR et al (2003) Raf-1 and Bcl-2 induce distinct and common pathways that contribute to breast cancer drug resistance. Clin Cancer Res off J Am Assoc Cancer Res 9(3):1161–1170
El-Ashry D, Miller DL, Kharbanda S, Lippman ME, Kern FG (1997) Constitutive Raf-1 kinase activity in breast cancer cells induces both estrogen-independent growth and apoptosis. Oncogene 15(4):423–435
Fillmore CM, Xu C, Desai PT et al (2015) EZH2 inhibition sensitizes BRG1 and EGFR mutant lung tumours to TopoII inhibitors. Nature 520(7546):239–242
Huang HN, Lin MC, Huang WC, Chiang YC, Kuo KT (2014) Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations and ZNF217 amplification in ovarian clear cell carcinoma. Modern Pathol off J U S Can Acad Pathol. 27(7):983–990
Huang WF, Liu AH, Zhao HJ, Dong HM, Liu LY, Cai SX (2015) BIM gene polymorphism lowers the efficacy of EGFR-TKIs in advanced nonsmall cell lung cancer with sensitive EGFR mutations: a systematic review and meta-analysis. Medicine 94(33):e1263
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520
Machado I, Navarro S, Llombart-Bosch A (2016) Ewing sarcoma and the new emerging Ewing-like sarcomas: (CIC and BCOR-rearranged-sarcomas). A systematic review. Histol Histopathol 31(11):1169–1181
Marino-Enriquez A, Fletcher CD (2014) Round cell sarcomas - biologically important refinements in subclassification. Int J Biochem Cell Biol 53:493–504
Meis-Kindblom JM, Stenman G, Kindblom LG (1996) Differential diagnosis of small round cell tumors. Semin Diagn Pathol 13(3):213–241
Nie W, Tao X, Wei H, Chen WS, Li B (2015) The BIM deletion polymorphism is a prognostic biomarker of EGFR-TKIs response in NSCLC: a systematic review and meta-analysis. Oncotarget 6(28):25696–25700
Pappo AS, Dirksen U (2018) Rhabdomyosarcoma, Ewing sarcoma, and other round cell sarcomas. J Clin Oncol off J Am Soc Clin Oncol 36(2):168–179
Pierron G, Tirode F, Lucchesi C et al (2012) A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet 44(4):461–466
Rabbitts TH (1994) Chromosomal translocations in human cancer. Nature 372(6502):143–149
Rajwanshi A, Srinivas R, Upasana G (2009) Malignant small round cell tumors. J Cytol 26(1):1–10
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, NY) 348(6230):124–128
Rosenberg JE, Hoffman-Censits J, Powles T et al (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 387(10031):1909–1920
Samartzis EP, Gutsche K, Dedes KJ, Fink D, Stucki M, Imesch P (2014) Loss of ARID1A expression sensitizes cancer cells to PI3K- and AKT-inhibition. Oncotarget 5(14):5295–5303
Sbaraglia M, Righi A, Gambarotti M, Dei Tos AP (2020) Ewing sarcoma and Ewing-like tumors. Virchows Archiv Int J Pathol 476(1):109–119
Sharma S, Kamala R, Nair D et al (2017) Round cell tumors: classification and Immunohistochemistry. Indian J Med Paediatr Oncol off J Indian Soc Med Paediatr Oncol 38(3):349–353
Shen J, Peng Y, Wei L et al (2015) ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov 5(7):752–767
Snyder A, Makarov V, Merghoub T et al (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371(23):2189–2199
Watanabe R, Ui A, Kanno S et al (2014) SWI/SNF factors required for cellular resistance to DNA damage include ARID1A and ARID1B and show interdependent protein stability. Can Res 74(9):2465–2475
Xia SJ, Barr FG (2005) Chromosome translocations in sarcomas and the emergence of oncogenic transcription factors. Eur J Cancer (oxford, England: 1990). 41(16):2513–2527
Funding
This work were supported by grants from the National Natural Science Foundation of China (82273449) and Medical and Health Science and Technology Project of Zhejiang Province (2024KY1039).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by ZW, The first draft of the manuscript was written by ZW and JY, published articles were reviewed by JH. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The author reports no conflicts of interest in this work.
Ethical approval
This study was approved by the ethics committee of the Second Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, People’s Republic of China). All clinical investigations were conducted in accordance with the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from the patient.
Consent to publish
The authors affirm that the patient provided informed consent for publication of the clinical data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Ye, J., Hu, J. et al. A rare Ewing-like small round cell tumor in prostate: a case report and literature review. J Cancer Res Clin Oncol 150, 110 (2024). https://doi.org/10.1007/s00432-023-05585-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-023-05585-2