Abstract
Purpose
We aimed to assess the predictive value of galectin-3 (Gal-3) in patients with non-small cell lung cancer (NSCLC) treated with immune checkpoint blockades (ICBs) therapy using both enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry (IHC).
Methods
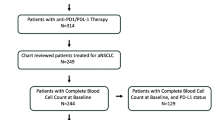
This retrospective study was conducted at Seoul National University Hospital. Patients with EGFR/ALK-wild-type advanced or metastatic NSCLC who received ICBs between December 2013 and December 2019 were enrolled. Patients with archived blood samples collected prior to ICB treatment were assigned to the ELISA cohort. In addition, those with tissue samples from sites of recurrence or metastasis were assigned to an IHC cohort. Then, we analyzed Gal-3 expression in both cohorts.
Results
Fifty-six patients in the ELISA cohort were grouped into low (N = 36) and high (N = 20) groups, using the mean Gal-3 ELISA level (13.24 pg/ml) as a cutoff. The high group demonstrated trends toward reduced progression-free survival (PFS) (0.9 vs. 3.7 months, p = 0.196) and significantly shorter overall survival (OS) (1.6 vs. 12.3 months, p = 0.018) than the low group. We categorized 94 patients in the IHC cohort into negative (N = 31) and positive (N = 63) groups based on Gal-3 IHC positivity. However, the median PFS (4.6 vs. 4.6 months for the negative vs. positive IHC group, respectively, p = 0.345) and OS (16.4 vs. 9.0 months, p = 0.137) were not significantly different.
Conclusion
High blood Gal-3 levels may predict inferior survival in patients with advanced or metastatic NSCLC treated with ICBs.





Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguilar EJ, Ricciuti B, Gainor JF et al (2019) Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol 30(10):1653–1659. https://doi.org/10.1093/annonc/mdz288
Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A (1997) Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res 57:5272–5276
Bendtsen SK, Perez-Penco M, Hübbe ML et al (2022) Peptide vaccination activating galectin-3-specific T cells offers a novel means to target galectin-3-expressing cells in the tumor microenvironment. Oncoimmunology 11:2026020. https://doi.org/10.1080/2162402X.2022.2026020
Blair BB, Funkhouser AT, Goodwin JL et al (2021) Increased circulating levels of galectin proteins in patients with breast, colon, and lung cancer. Cancers (basel). https://doi.org/10.3390/cancers13194819
Brahmer JR (2013) Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol 31:1021–1028. https://doi.org/10.1200/JCO.2012.45.8703
Capalbo C, Scafetta G, Filetti M, Marchetti P, Bartolazzi A (2019) Predictive biomarkers for checkpoint inhibitor-based immunotherapy: The galectin-3 signature in NSCLCs. Int J Mol Sci. https://doi.org/10.3390/ijms20071607
Cervantes-Alvarez E, la Rosa NL, la Mora MS et al (2022) Galectin-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci Rep 12:1856. https://doi.org/10.1038/s41598-022-05968-4
Chang L, Chang M, Chang HM, Chang F (2018) Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol 26:e15–e21. https://doi.org/10.1097/PAI.0000000000000575
Curti BD, Koguchi Y, Leidner RS et al (2021) Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J Immunother Cancer 9:e002371. https://doi.org/10.1136/jitc-2021-002371
Ghiringhelli F, Doucet L, Barre P et al (2022) GALLANT-1: Galectin-3 (Gal-3) inhibitor GB1211 plus atezolizumab (atezo) in patients with non–small cell lung cancer (NSCLC)—a randomized, double-blind trial. J Clin Oncol. https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS9152
Gonnermann D, Oberg HH, Lettau M et al (2020) Galectin-3 released by pancreatic ductal adenocarcinoma suppresses γδ T cell proliferation but not their cytotoxicity. Front Immunol 11:1328. https://doi.org/10.3389/fimmu.2020.01328
Gordon-Alonso M, Hirsch T, Wildmann C, van der Bruggen P (2017) Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat Commun 8:793. https://doi.org/10.1038/s41467-017-00925-6
Hellmann MD, Callahan MK, Awad MM et al (2018) Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 33:853-861.e4. https://doi.org/10.1016/j.ccell.2018.04.001
Hirabayashi J, Kasai K (1993) The family of metazoan metal-independent beta-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 3:297–304. https://doi.org/10.1093/glycob/3.4.297
Ho JE, Liu C, Lyass A et al (2012) Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 60:1249–1256. https://doi.org/10.1016/j.jacc.2012.04.053
Honjo Y, Inohara H, Akahani S, Yoshii T, Takenaka Y, Yoshida J, Hattori K, Tomiyama Y, Raz A, Kubo T (2000) Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin Cancer Res 6:4635–4640
Honjo Y, Nangia-Makker P, Inohara H, Raz A (2001) Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res 7:661–668
Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res 6:1389–1393
Kao MW, Su YC, Liang PI, Wu YY, Hong TM (2020) Low galectin-3 expression level in primary tumors is associated with metastasis in T1 lung adenocarcinoma. J Clin Med 9:1990. https://doi.org/10.3390/jcm9061990
Kataoka Y, Igarashi T, Ohshio Y, Fujita T, Hanaoka J (2019) Predictive importance of galectin-3 for recurrence of non-small cell lung cancer. Gen Thorac Cardiovasc Surg 67:704–711. https://doi.org/10.1007/s11748-019-01074-x
Kim HR, Lin HM, Biliran H, Raz A (1999) Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res 59:4148–4154
Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E (2015) Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol Res 3:412–423. https://doi.org/10.1158/2326-6066.CIR-14-0150
Kusuhara S, Igawa S, Ichinoe M et al (2021) Prognostic significance of galectin-3 expression in patients with resected NSCLC treated with platinum-based adjuvant chemotherapy. Thorac Cancer 12:1570–1578. https://doi.org/10.1111/1759-7714.13945
Lantuejoul S, Sound-Tsao M, Cooper WA et al (2020) PD-L1 testing for lung cancer in 2019: perspective from the IASLC pathology committee. J Thorac Oncol 15:499–519. https://doi.org/10.1016/j.jtho.2019.12.107
Mathieu A, Saal I, Vuckovic A, Ransy V, Vereerstraten P, Kaltner H, Gabius HJ, Kiss R, Decaestecker C, Salmon I, Remmelink M (2005) Nuclear galectin-3 expression is an independent predictive factor of recurrence for adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol 18:1264–1271. https://doi.org/10.1038/modpathol.3800416
Park S, Ock CY, Kim H et al (2022) JCO2102010 Artificial intelligence-powered spatial analysis of tumor-infiltrating lymphocytes as complementary biomarker for immune checkpoint inhibition in non-small-cell lung cancer. J Clin Oncol 40:1916–1928. https://doi.org/10.1200/JCO.21.02010
Ramos-Martínez JC, Altamirano-Gómez G, Ramos-Martínez I, Valencia J, Hernández-Zimbrón L, Hernández-Juárez J, Echeverría-Vásquez P, Hernández-González LL, Pérez-Campos E, Pérez-Campos Mayoral L, Ramos-Martínez E (2022) Prognostic value of galectin expression in patients with breast cancer: systematic review and meta-analysis. Clin Breast Cancer 22:399–409. https://doi.org/10.1016/j.clbc.2021.12.011
Ratcliffe MJ, Sharpe A, Midha A et al (2017) Agreement between programmed cell death Ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res 23:3585–3591. https://doi.org/10.1158/1078-0432.CCR-16-2375
Ruvolo PP (2016) Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta 1863:427–437. https://doi.org/10.1016/j.bbamcr.2015.08.008
Stillman BN, Hsu DK, Pang M et al (2006) Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 176:778–789. https://doi.org/10.4049/jimmunol.176.2.778
van den Brûle FA, Waltregny D, Liu FT, Castronovo V (2000) Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer 89:361–367. https://doi.org/10.1002/1097-0215(20000720)89:4%3c361::aid-ijc8%3e3.0.co;2-u
Vuong L, Kouverianou E, Rooney CM et al (2019) An orally active galectin-3 antagonist inhibits lung adenocarcinoma growth and augments response to PD-L1 blockade. Cancer Res 79:1480–1492. https://doi.org/10.1158/0008-5472.CAN-18-2244
Wang Y, Liu S, Tian Y, Wang Y, Zhang Q, Zhou X, Meng X, Song N (2018) Prognostic role of galectin-3 expression in patients with solid tumors: a meta-analysis of 36 eligible studies. Cancer Cell Int 18:172. https://doi.org/10.1186/s12935-018-0668-y
Yi N, Zhao X, Ji J et al (2020) Serum galectin-3 as a biomarker for screening, early diagnosis, prognosis and therapeutic effect evaluation of pancreatic cancer. J Cell Mol Med 24:11583–11591. https://doi.org/10.1111/jcmm.15775
Zeng N, Wang A, Zhong C et al (2019) Association of serum galectin-3 with risks of death and vascular events in acute ischaemic stroke patients: the role of hyperglycemia. Eur J Neurol 26:415–421. https://doi.org/10.1111/ene.13856
Zhang H, Liu P, Zhang Y et al (2021) Inhibition of galectin-3 augments the antitumor efficacy of PD-L1 blockade in non-small-cell lung cancer. FEBS Open Bio 11:911–920. https://doi.org/10.1002/2211-5463.13088
Funding
This study was supported by grant number 04-2021-3110 from the SNUH Research Fund.
Author information
Authors and Affiliations
Contributions
JSK and DWK conceived and designed the analysis. JSK, SK, and JK collected the data. JSK, SK, JK, and DWK contributed data or analysis tools. JSK, SK, JK, and DWK performed the analysis. JSK, SK, JK, MK, BK, TMK, BL, and DWK wrote the paper. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. D-W Kim’s Institution (Seoul National University Hospital) received grants from Alpha Biopharma, Amgen, Astrazeneca/Medimmune, Boehringer-Ingelheim, BMS, Bridge BioTherapeutics, Chong Keun Dang, Daiichi-Sankyo, GSK, Hanmi, InnoN, Janssen, Merck, Merus, Mirati Therapeutics, MSD, Novartis, ONO Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, and Yuhan. In addition, Dr. D-W Kim received travel support for advisory board meeting attendance from Amgen and Daiichi-Sankyo. All these COIs are outside of the submitted work. Dr. B Lindmark is an employee of Galecto, Inc. Dr. TM Kim’s institution received clinical trial budgets from AstraZeneca/MedImmune, Bayer, Boehringer-Ingelheim, Boryung, Genmab, Hanmi, Janssen, Merck Serono, Merck Sharp & Dohme Corp, Novartis, REGENERON, Roche/Genentech, Sanofi, and Takeda. Dr. TM Kim received advisory or consulting fees from AstraZeneca, Hanmi, Janssen, Novartis, Roche/Genentech, and Takeda. In addition, Dr. TM Kim received research grant from AstraZeneca-KHIDI outside this work. Dr. M Kim received advisory or consulting fees from Merck Sharp & Dohme Corp, Ipsen, Bristol-Myers Squibb/Ono Pharmaceutical, Eisai, and Yuhan. No conflicts of interest are disclosed by the other authors.
Ethical approval
The study was conducted in accordance with the Principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of the SNUH (IRB No. H-2006-146-1134).
Consent to participate
Informed consent for secondary analysis was obtained from all the individual participants included in the study before participation.
Consent to publish
The consent to publish is not applicable as the manuscript does not contain any identifiable information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J.S., Kim, S., Koh, J. et al. Predictive role of galectin-3 for immune checkpoint blockades (ICBs) in advanced or metastatic non-small cell lung cancer: a potential new marker for ICB resistance. J Cancer Res Clin Oncol 149, 2355–2365 (2023). https://doi.org/10.1007/s00432-022-04275-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04275-9