Abstract
Background
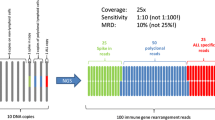
Next-generation sequencing (NGS) is an emerging technology that can comprehensively assess the diversity of the immune system. We explored the feasibility of NGS in detecting minimal residual disease (MRD) in childhood acute lymphoblastic leukemia (ALL) based on immunoglobulin and T cell receptor.
Methods
Bone marrow samples were collected pre- and post-treatment with pediatric ALL admitted to Shenzhen Children's Hospital from February 1st, 2020 to January 31st, 2021. We analyzed the MRD detected by NGS, multiparametric flow cytometry (MFC) and real-time quantitative PCR (RQ–PCR), and analyzed risk factors of positive NGS–MRD at the end of B-ALL induction chemotherapy.
Results
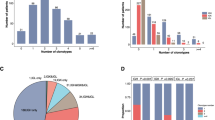
A total of paired 236 bone marrow samples were collected from 64 children with ALL (58 B-ALL and 6 T-ALL). The decrease in the clonal rearrangement frequency of IGH, IGK, and IGL was generally consistent after treatment. Positive MRD was detected in 57.5% (77/134) of B-ALL and 80% (12/15) of T-ALL by NGS after chemotherapy, which was higher than those detected by MFC and RQ–PCR. In B-ALL patients, MRD results detected by NGS were consistent with MFC (r = 0.708, p < 0.001) and RQ–PCR (r = 0.618, p < 0.001). At the end of induction, NGS–MRD of 40.4% B-ALL was > 0.01% and multivariate analysis indicated that ≧2 clonal rearrangement sequences before treatment were an independent factor of negative NGS–MRD.
Conclusions
NGS is more sensitive than MFC and RQ–PCR for MRD measurement. B-ALL children with ≧2 clonal rearrangements detected by NGS before treatment are difficult to switch to negative MRD after chemotherapy.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Bader P et al (2015) Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol 33:1275–1284. https://doi.org/10.1200/JCO.2014.58.4631
Berry DA et al (2017) Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia. AMeta-analysis. 3:e170580
Borowitz MJ et al (2008) Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood 111:5477–5485. https://doi.org/10.1182/blood-2008-01-132837
Brüggemann M et al (2019) Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study. Leukemia 33:2241–2253. https://doi.org/10.1038/s41375-019-0496-7
Ceppi F, Rizzati F, Colombini A, Conter V, Cazzaniga G (2021) Utilizing the prognostic impact of minimal residual disease in treatment decisions for pediatric acute lymphoblastic leukemia. Expert Rev Hematol 14:795–807. https://doi.org/10.1080/17474086.2021.1967137
Cheng S, Inghirami G, Cheng S, Tam W (2018) Simple deep sequencing-based post-remission MRD surveillance predicts clinical relapse in B-ALL. J Hematol Oncol 11:105. https://doi.org/10.1186/s13045-018-0652-y
Coccaro N, Anelli L, Zagaria A, Specchia G, Albano F (2019) Next-generation sequencing in acute lymphoblastic leukemia. Int J Mol Sci. https://doi.org/10.3390/ijms20122929
Contreras Yametti GP, Ostrow TH, Jasinski S, Raetz EA, Carroll WL, Evensen NA (2021) Minimal residual disease in acute lymphoblastic leukemia: current practice and future directions. Cancers (Basel). https://doi.org/10.3390/cancers13081847
Faham M et al (2012) Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 120:5173–5180. https://doi.org/10.1182/blood-2012-07-444042
Knecht H et al (2019) Quality control and quantification in IG/TR next-generation sequencing marker identification: protocols and bioinformatic functionalities by EuroClonality-NGS. Leukemia 33:2254–2265. https://doi.org/10.1038/s41375-019-0499-4
Kotrova M et al (2015) The predictive strength of next-generation sequencing MRD detection for relapse compared with current methods in childhood ALL. Blood 126:1045–1047. https://doi.org/10.1182/blood-2015-07-655159
Kotrova M et al (2017) Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant 52:962–968
Kotrova M et al (2018) Next-generation amplicon TRB locus sequencing can overcome limitations of flow-cytometric Vβ expression analysis and confirms clonality in all T-cell prolymphocytic leukemia cases. Cytometry A 93:1118–1124. https://doi.org/10.1002/cyto.a.23604
Kruse A et al (2020) Minimal residual disease detection in acute lymphoblastic leukemia. Int J Mol Sci. https://doi.org/10.3390/ijms21031054
Ladetto M et al (2014) Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 28:1299–1307. https://doi.org/10.1038/leu.2013.375
Langerak AW et al (2012) EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 26:2159–2171. https://doi.org/10.1038/leu.2012.246
Lazic J, Tosic N, Dokmanovic L, Krstovski N, Rodic P, Pavlovic S, Janic D (2010) Clinical features of the most common fusion genes in childhood acute lymphoblastic leukemia. Med Oncol 27:449–453. https://doi.org/10.1007/s12032-009-9232-x
Logan AC et al (2014) Immunoglobulin and T cell receptor gene high-throughput sequencing quantifies minimal residual disease in acute lymphoblastic leukemia and predicts post-transplantation relapse and survival. Biol Blood Marrow Transplant 20:1307–1313. https://doi.org/10.1016/j.bbmt.2014.04.018
Modvig S et al (2019) Minimal residual disease quantification by flow cytometry provides reliable risk stratification in T-cell acute lymphoblastic leukemia. Leukemia 33:1324–1336. https://doi.org/10.1038/s41375-018-0307-6
Monter A, Nomdedéu JF (2019) ClonoSEQ assay for the detection of lymphoid malignancies. Expert Rev Mol Diagn 19:571–578. https://doi.org/10.1080/14737159.2019.1627877
Pui CH et al (2015) Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol 16:465–474. https://doi.org/10.1016/S1470-2045(15)70082-3
Pui CH et al (2017) Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with response-adapted therapy. Leukemia 31:333–339. https://doi.org/10.1038/leu.2016.234
Pulsipher MA et al (2015) IgH-V (D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood 125:3501–3508. https://doi.org/10.1182/blood-2014-12-615757
Qin X, Zhang MY, Liu WJ (2018) Application of minimal residual disease monitoring in pediatric patients with acute lymphoblastic leukemia. Eur Rev Med Pharmacol Sci 22:6885–6895. https://doi.org/10.26355/eurrev_201810_16158
Roshal M, Fromm JR, Winter S, Dunsmore K, Wood BL (2010) Immaturity associated antigens are lost during induction for T cell lymphoblastic leukemia: implications for minimal residual disease detection. Cytometry B Clin Cytom 78:139–146. https://doi.org/10.1002/cyto.b.20511
Sala Torra O et al (2017) Next-generation sequencing in adult B cell acute lymphoblastic leukemia patients. Biol Blood Marrow Transplant 23:691–696. https://doi.org/10.1016/j.bbmt.2016.12.639
Sekiya Y et al (2017) Clinical utility of next-generation sequencing-based minimal residual disease in paediatric B-cell acute lymphoblastic leukaemia. Br J Haematol 176:248–257. https://doi.org/10.1111/bjh.14420
Stow P et al (2010) Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood 115:4657–4663. https://doi.org/10.1182/blood-2009-11-253435
van den Brand M et al (2021) Next-generation sequencing-based clonality assessment of ig gene rearrangements: a multicenter validation study by euroclonality-NGS. J Mol Diagn 23:1105–1115. https://doi.org/10.1016/j.jmoldx.2021.06.005
van Dongen JJ, van der Velden VH, Brüggemann M, Orfao A (2015) Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood 125:3996–4009. https://doi.org/10.1182/blood-2015-03-580027
Wood B et al (2018) Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood 131:1350–1359. https://doi.org/10.1182/blood-2017-09-806521
Wu D et al (2012) High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med 4:134ra63. https://doi.org/10.1126/scitranslmed.3003656
Wu D et al (2014) Detection of minimal residual disease in B lymphoblastic leukemia by high-throughput sequencing of IGH. Clin Cancer Res 20:4540–4548. https://doi.org/10.1158/1078-0432.CCR-13-3231
Acknowledgements
We are grateful to all the colleagues in Hematology and Oncology department of Shenzhen Children's Hospital for supporting this work in the clinical practice. We thank Mingfeng Bai and Cheng Chen from Institute of Health and Medical Technology, Hefei Institutes of Physical Science, Chinese Academy of Sciences for revising the manuscript. This project was supported by Sanming Project of Medicine in Shenzhen (SZSM201512033), Guangdong Medical Science and Technology Research Project (A2020101), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP012), Shenzhen Key Medical Discipline Construction Fund (SZXK034), and Shenzhen Healthcare Research Project (SZLY2018015).
Funding
This work was supported by Sanming Project of Medicine in Shenzhen (SZSM201512033), Guangdong Medical Science and Technology Research Project (A2020101), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP012), Shenzhen Key Medical Discipline Construction Fund (SZXK034), and Shenzhen Healthcare Research Project (SZLY2018015).
Author information
Authors and Affiliations
Contributions
(I) conception and design: Huirong Mai, Qin Li, Sixi Liu (II) Administrative support: Sixi Liu; (III) Provision of study material or patients: Guobing Wang, Ying Wang, Shilin Liu, Xue Tang, Fen Chen; (IV) Collection and assembly of data: Guichi Zhou, Yi Liu, Tonghui Li, Lulu Wang, Chunyan Wang, Feiqiu Wen; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shenzhen Children's Hospital (June 11, 2021/No. 202105102).
Consent to participate
All cases obtained written informed consent from their parents.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mai, H., Li, Q., Wang, G. et al. Clinical application of next-generation sequencing-based monitoring of minimal residual disease in childhood acute lymphoblastic leukemia. J Cancer Res Clin Oncol 149, 3259–3266 (2023). https://doi.org/10.1007/s00432-022-04151-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04151-6