Abstract
Purpose
Assessing the downstaging effects of neoadjuvant chemotherapy (NACT) in patients with locally advanced nasopharyngeal carcinoma (LANPC) and predicting response to treatment remain challenging. The present study aimed to evaluate the long-term prognosis of downstaging after NACT in patients with LANPC and to investigate the prognostic value of post-NACT tumor downstaging on treatment outcomes in the era of concurrent chemoradiotherapy (CCRT).
Methods
This retrospective study included 226 patients with stage III (n = 188) and IVA (n = 38) NPC admitted to Haikou People’s Hospital between 1 October 2009 and 1 October 2012. The patients were grouped as downstaging or no after NACT. Overall survival (OS), locoregional failure-free survival (LFFS), and distant failure-free survival (DFFS) were analyzed.
Results
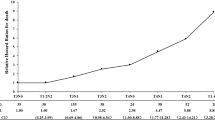
Among 226 patients, 196 (86.7%) were in the downstaging group and 30 (13.3%) were in the non-downstaging group. The longest follow-up was 76 months, and the median was 45 months. The 3-year OS rates of the downstaging group and non-downstaging group were 91.0% (95% CI 0.89–0.93) and 69.5% (95% CI 0.66–0.72) (P = 0.005). The 5-year OS rates were 81.6% (95% CI 0.78–0.86) and 53.3% (95% CI 0.49–0.61) (P = 0.001). N downstaging (3-year OS, HR 0.491, 95% CI 0.221–0.881, P = 0.022; 5-year OS, HR = 0.597, 95% CI 0.378–0.878, P = 0.021) was independently associated with OS.
Conclusion
In the treatment of LANPC, the patients with downstaging after NACT have a better prognosis than those without downstaging. This study suggests that NACT can improve the prognosis for patients with LANPC if there is downstaging.


Similar content being viewed by others
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, Qian CN, Zhao C, Xiang YQ, Zhang XP, Lin ZX, Li WX, Liu Q, Qiu F, Sun R, Chen QY, Huang PY, Luo DH, Hua YJ, Wu YS, Lv X, Wang L, Xia WX, Tang LQ, Ye YF, Chen MY, Guo X, Hong MH (2017) Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer 75:14–23. https://doi.org/10.1016/j.ejca.2016.12.039
Chan AT (2010) Nasopharyngeal carcinoma. Ann Oncol 21(suppl 7):308–312. https://doi.org/10.1093/annonc/mdq277
Chan AT, Gregoire V, Lefebvre JL, Licitra L, Hui EP, Leung SF, Felip E, & Group E-E-EGW (2012) Nasopharyngeal cancer: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii833–vii885. https://doi.org/10.1093/annonc/mds266
Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, Wee JTS, Whitley AC, Yi JL, Yom SS, Chan ATC, Hu CS, Lang JY, Le QT, Lee AWM, Lee N, Lin JC, Ma B, Morgan TJ, Shah J, Sun Y, Ma J (2021) Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II–IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol 39:840–859. https://doi.org/10.1200/JCO.20.03237
Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, Yang CS (2001) Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 345:1877–1882. https://doi.org/10.1056/NEJMoa011610
Chua MLK, Wee JTS, Hui EP, Chan ATC (2016) Nasopharyngeal carcinoma. Lancet 387:1012–1024. https://doi.org/10.1016/S0140-6736(15)00055-0
Davies AR, Gossage JA, Zylstra J, Mattsson F, Lagergren J, Maisey N, Smyth EC, Cunningham D, Allum WH, Mason RC (2014) Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 32:2983–2990. https://doi.org/10.1200/JCO.2014.55.9070
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
NCCN Guidelines (2021) NCCN clinical practice guidelines in Oncology, Head and Neck Cancers. Version 2.2021. National Comprehensive Cancer Network, Fort Washington
Hao Y, Liu Y, Ishibashi H, Wakama S, Nishino E, Yonemura Y (2019) Downstaging of lymph node metastasis after neoadjuvant intraperitoneal and systemic chemotherapy in gastric carcinoma with peritoneal metastasis. Eur J Surg Oncol 45:1493–1497. https://doi.org/10.1016/j.ejso.2019.03.011
Jiang CY, Wang J, Chen ZM (2020) Comparison of the prognosis of advanced T and N stage in patients with nasopharyngeal carcinoma. Chin J Clin Oncol 47:780–783
Kong L, Zhang Y, Hu C, Guo Y, Lu JJ (2017) Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: final results of 2 parallel phase 2 clinical trials. Cancer 123:2258–2267. https://doi.org/10.1002/cncr.30566
Lacas B, Bourhis J, Overgaard J, Zhang Q, Gregoire V, Nankivell M, Zackrisson B, Szutkowski Z, Suwinski R, Poulsen M, O’Sullivan B, Corvo R, Laskar SG, Fallai C, Yamazaki H, Dobrowsky W, Cho KH, Beadle B, Langendijk JA, Viegas CMP, Hay J, Lotayef M, Parmar MKB, Auperin A, van Herpen C, Maingon P, Trotti AM, Grau C, Pignon JP, Blanchard P, Group MC (2017) Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol 18:1221–1237. https://doi.org/10.1016/S1470-2045(17)30458-8
Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H, Corry J, Grau C, Gregoire V, Harrington KJ, Hu CS, Kwong DL, Langendijk JA, Le QT, Lee NY, Lin JC, Lu TX, Mendenhall WM, O’Sullivan B, Ozyar E, Peters LJ, Rosenthal DI, Soong YL, Tao Y, Yom SS, Wee JT (2018) International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol 126:25–36. https://doi.org/10.1016/j.radonc.2017.10.032
Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, Chen XZ, Li JG, Zhu XD, Hu CS, Xu XY, Chen YY, Hu WH, Guo L, Mo HY, Chen L, Mao YP, Sun R, Ai P, Liang SB, Long GX, Zheng BM, Feng XL, Gong XC, Li L, Shen CY, Xu JY, Guo Y, Chen YM, Zhang F, Lin L, Tang LL, Liu MZ, Ma J, Sun Y (2019) Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer 145:295–305. https://doi.org/10.1002/ijc.32099
Liu F, Jin T, Liu L, Xiang Z, Yan R, Yang H (2018a) The role of concurrent chemotherapy for stage II nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a systematic review and meta-analysis. PLoS ONE 13:e0194733. https://doi.org/10.1371/journal.pone.0194733
Liu L, Fei Z, Chen M, Zhao L, Su H, Gu D, Lin B, Cai X, Lu L, Gao M, Ye X, Jin X, Xie C (2018b) Induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus volumetric modulated arc therapy alone in the treatment of stage II–IVB nasopharyngeal carcinoma patients: a retrospective controlled study. Radiat Oncol 13:148. https://doi.org/10.1186/s13014-018-1092-0
Pisani P, Airoldi M, Allais A, Aluffi Valletti P, Battista M, Benazzo M, Briatore R, Cacciola S, Cocuzza S, Colombo A, Conti B, Costanzo A, Della Vecchia L, Denaro N, Fantozzi C, Galizia D, Garzaro M, Genta I, Iasi GA, Krengli M, Landolfo V, Lanza GV, Magnano M, Mancuso M, Maroldi R, Masini L, Merlano MC, Piemonte M, Pisani S, Prina-Mello A, Prioglio L, Rugiu MG, Scasso F, Serra A, Valente G, Zannetti M, Zigliani A (2020) Metastatic disease in head & neck oncology. Acta Otorhinolaryngol Ital 40:S1–S86. https://doi.org/10.14639/0392-100X-suppl.1-40-2020
Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L (2016) RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer 62:132–137. https://doi.org/10.1016/j.ejca.2016.03.081
Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, Chen XZ, Li JG, Zhu XD, Hu CS, Xu XY, Chen YY, Hu WH, Guo L, Mo HY, Chen L, Mao YP, Sun R, Ai P, Liang SB, Long GX, Zheng BM, Feng XL, Gong XC, Li L, Shen CY, Xu JY, Guo Y, Chen YM, Zhang F, Lin L, Tang LL, Liu MZ, Ma J (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17:1509–1520. https://doi.org/10.1016/S1470-2045(16)30410-7
Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK, Ng QS, Tan D, Ong WS, Tan SH, Yip C, Quah D, Soo KC, Wee J (2015) Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 91:952–960. https://doi.org/10.1016/j.ijrobp.2015.01.002
Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, Lo KW (2014) Etiological factors of nasopharyngeal carcinoma. Oral Oncol 50:330–338. https://doi.org/10.1016/j.oraloncology.2014.02.006
Wang M, Tian H, Li G, Ge T, Liu Y, Cui J, Han F (2016) Significant benefits of adding neoadjuvant chemotherapy before concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Oncotarget 7:48375–48390. https://doi.org/10.18632/oncotarget.10237
Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, Chua ET, Yang E, Lee KM, Fong KW, Tan HS, Lee KS, Loong S, Sethi V, Chua EJ, Machin D (2005) Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 23:6730–6738. https://doi.org/10.1200/JCO.2005.16.790
Wei Z, Zhang Z, Luo J, Li N, Peng X (2019) Induction chemotherapy plus IMRT alone versus induction chemotherapy plus IMRT-based concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a retrospective cohort study. J Cancer Res Clin Oncol 145:1857–1864. https://doi.org/10.1007/s00432-019-02925-z
Xu C, Sun R, Tang LL, Chen L, Li WF, Mao YP, Zhou GQ, Guo R, Lin AH, Sun Y, Ma J, Hu WH (2018) Role of sequential chemoradiotherapy in stage II and low-risk stage III–IV nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy: a propensity score-matched analysis. Oral Oncol 78:37–45. https://doi.org/10.1016/j.oraloncology.2018.01.008
Yao JJ, Yu XL, Zhang F, Zhang WJ, Zhou GQ, Tang LL, Mao YP, Chen L, Ma J, Sun Y (2017) Radiotherapy with neoadjuvant chemotherapy versus concurrent chemoradiotherapy for ascending-type nasopharyngeal carcinoma: a retrospective comparison of toxicity and prognosis. Chin J Cancer 36:26. https://doi.org/10.1186/s40880-017-0195-6
Yin ZZ, Wang YY, Zhang XM (2018) Treatment outcomes of different induction chemotherapy regimens combined with in-tensity-modulated radiotherapy in nasopharyngeal carcinoma. Chin J Clin Oncol 45:179–184
Yuan C, Xu XH, Luo SW, Wang L, Sun M, Ni LH, Xu L, Wang XL, Zeng G (2018) Which neoadjuvant chemotherapy regimen should be recommended for patients with advanced nasopharyngeal carcinoma?: a network meta-analysis. Medicine (baltimore) 97:e11978. https://doi.org/10.1097/MD.0000000000011978
Zhang B, Hu Y, Xiong RH, Pan YF, Xu QL, Kong XY, Cai R, Chen QQ, Tang HY, Jiang W (2017) Matched analysis of induction chemotherapy plus chemoradiotherapy versus induction chemotherapy plus radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a multicenter study. Oncotarget 8:14078–14088. https://doi.org/10.18632/oncotarget.13285
Zhang B, Li MM, Chen WH, Zhao JF, Chen WQ, Dong YH, Gong X, Chen QY, Zhang L, Mo XK, Luo XN, Tian J, Zhang SX (2019) Association of chemoradiotherapy regimens and survival among patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. JAMA Netw Open 2:e1913619. https://doi.org/10.1001/jamanetworkopen.2019.13619
Zhong Q, Zhu X, Li L, Qu S, Liang Z, Zeng F, Pan X (2017) IMRT combined with concurrent chemotherapy plus adjuvant chemotherapy versus IMRT combined with concurrent chemotherapy alone in patients with nasopharyngeal carcinoma. Oncotarget 8:39683–39694. https://doi.org/10.18632/oncotarget.14799
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ZL, GC, and SC. The first draft of the manuscript was written by WW, HW, YL, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
This study was approved by the Ethics Committee of Haikou People’s Hospital (Ethics Review Approval Number: 2009-(ER)-082).
Consent to participate
The requirement for informed consent was waived by the committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, W., Peng, S., Wu, H. et al. Association of tumor downstaging after neoadjuvant chemotherapy with survival in patients with locally advanced nasopharyngeal carcinoma: a retrospective cohort study. J Cancer Res Clin Oncol 147, 2913–2922 (2021). https://doi.org/10.1007/s00432-021-03690-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03690-8