Abstract
Purpose
To investigate the role of HER2 positivity in prognosis and unresponsiveness to anti-EGFR therapy for colorectal cancer.
Methods
Patients who underwent primary CRC tumor resection were included. HER2 status of CRC was confirmed by immunohistochemistry and fluorescence in situ hybridization tests. Comparison of survival analysis between HER2 positivity and negativity was evaluated by a stratified log-rank test and summarized with the use of Kaplan–Meier and Cox proportional hazards methods. The treatment effects of cetuximab were further compared in full subgroup analyses.
Result
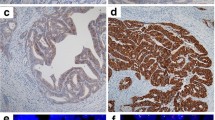
1240 patients were enrolled, including 763 with stage I-III CRC and 477 with stage IV CRC. 57 (4.6%) CRC patients presented HER2 positivity in the entire cohort. The survival analysis showed that patients with HER2 positivity had significantly worse disease-free survival and overall survival in stage III and IV CRC. The multivariable analysis also confirmed that HER2 positivity was a significantly independent risk factor in stage III and IV CRC. Univariate and multivariable survival analysis showed no prognostic significance of HER2 positivity in stage I–II CRC patients. Prespecified subgroup analysis showed no favorable trends in progression-free survival and overall survival for cetuximab in the patients with HER2 positivity, KRAS/NRAS/BRAF wild-type metastatic CRC (interaction P values = 0.005 and 0.014).
Conclusion
For stage III and IV CRC patients, HER2 positivity was confirmed as an independent prognostic risk factor. It could help predict the unresponsiveness to anti-EGFR therapy for metastatic CRC.





Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Allegra CJ, Rumble RB, Hamilton SR, Mangu PB, Roach N, Hantel A, Schilsky RL (2016) Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American society of clinical oncology provisional clinical opinion update 2015. J Clin Oncol 34(2):179–185. https://doi.org/10.1200/JCO.2015.63.9674
Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Trusolino L (2011) A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 1(6):508–523. https://doi.org/10.1158/2159-8290.CD-11-0109
Conradi LC, Styczen H, Sprenger T, Wolff HA, Rodel C, Nietert M, Liersch T (2013) Frequency of HER-2 positivity in rectal cancer and prognosis. Am J Surg Pathol 37(4):522–531. https://doi.org/10.1097/PAS.0b013e318272ff4d
De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S (2011) KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol 12(6):594–603. https://doi.org/10.1016/S1470-2045(10)70209-6
Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J (2017) Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 17(2):79–92. https://doi.org/10.1038/nrc.2016.126
Greally M, Kelly CM, Cercek A (2018) HER2: an emerging target in colorectal cancer. Curr Probl Cancer 42(6):560–571. https://doi.org/10.1016/j.currproblcancer.2018.07.001
Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Aguirre-Ghiso JA (2016) Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 540(7634):588–592. https://doi.org/10.1038/nature20609
Ingold Heppner B, Behrens HM, Balschun K, Haag J, Kruger S, Becker T, Rocken C (2014) HER2/neu testing in primary colorectal carcinoma. Br J Cancer 111(10):1977–1984. https://doi.org/10.1038/bjc.2014.483
Kapitanovic S, Radosevic S, Kapitanovic M, Andelinovic S, Ferencic Z, Tavassoli M, Spaventi R (1997) The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology 112(4):1103–1113. https://doi.org/10.1016/s0016-5085(97)70120-3
Keum N, Giovannucci E (2019) Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 16(12):713–732. https://doi.org/10.1038/s41575-019-0189-8
Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, Ghaedi K (2019) Signaling pathways involved in colorectal cancer progression. Cell Biosci 9:97. https://doi.org/10.1186/s13578-019-0361-4
Kruszewski WJ, Rzepko R, Ciesielski M, Szefel J, Zielinski J, Szajewski M, Wojtacki J (2010) Expression of HER2 in colorectal cancer does not correlate with prognosis. Dis Markers 29(5):207–212. https://doi.org/10.3233/DMA-2010-0742
Lupo B, Sassi F, Pinnelli M, Galimi F, Zanella ER, Vurchio V, Trusolino L (2020) Colorectal cancer residual disease at maximal response to EGFR blockade displays a druggable Paneth cell-like phenotype. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aax8313
Mangiapane LR, Nicotra A, Turdo A, Gaggianesi M, Bianca P, Di Franco S, Stassi G (2021) PI3K-driven HER2 expression is a potential therapeutic target in colorectal cancer stem cells. Gut. https://doi.org/10.1136/gutjnl-2020-323553
Mao Y, Feng Q, Zheng P, Yang L, Zhu D, Chang W, Xu J (2018) Low tumor infiltrating mast cell density confers prognostic benefit and reflects immunoactivation in colorectal cancer. Int J Cancer 143(9):2271–2280. https://doi.org/10.1002/ijc.31613
Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Hainsworth J (2019) Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 20(4):518–530. https://doi.org/10.1016/S1470-2045(18)30904-5
Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A (2014) Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov 4(11):1269–1280. https://doi.org/10.1158/2159-8290.CD-14-0462
Morano F, Corallo S, Lonardi S, Raimondi A, Cremolini C, Rimassa L, Pietrantonio F (2019) Negative hyperselection of patients With RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol 37(33):3099–3110. https://doi.org/10.1200/JCO.19.01254
Nguyen LH, Goel A, Chung DC (2020) Pathways of colorectal carcinogenesis. Gastroenterology 158(2):291–302. https://doi.org/10.1053/j.gastro.2019.08.059
Nowak JA (2020) HER2 in colorectal carcinoma: are we there yet? Surg Pathol Clin 13(3):485–502. https://doi.org/10.1016/j.path.2020.05.007
Oh DY, Bang YJ (2020) HER2-targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol 17(1):33–48. https://doi.org/10.1038/s41571-019-0268-3
Osako T, Miyahara M, Uchino S, Inomata M, Kitano S, Kobayashi M (1998) Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology 55(6):548–555. https://doi.org/10.1159/000011911
Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, Quirke P (2016) HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol 238(4):562–570. https://doi.org/10.1002/path.4679
Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Siena S (2016) Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 17(6):738–746. https://doi.org/10.1016/S1470-2045(16)00150-9
Sawada K, Nakamura Y, Yamanaka T, Kuboki Y, Yamaguchi D, Yuki S, Fujii S (2018) Prognostic and predictive value of HER2 amplification in patients with metastatic colorectal cancer. Clin Colorectal Cancer 17(3):198–205. https://doi.org/10.1016/j.clcc.2018.05.006
Song Z, Deng Y, Zhuang K, Li A, Liu S (2014) Immunohistochemical results of HER2/neu protein expression assessed by rabbit monoclonal antibodies SP3 and 4B5 in colorectal carcinomas. Int J Clin Exp Pathol 7(7): 4454–4460. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25120833
Sveen A, Kopetz S, Lothe RA (2020) Biomarker-guided therapy for colorectal cancer: strength in complexity. Nat Rev Clin Oncol 17(1):11–32. https://doi.org/10.1038/s41571-019-0241-1
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321(3):288–300. https://doi.org/10.1001/jama.2018.19323
Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Janne PA (2011) Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 3(99):99ra86. https://doi.org/10.1126/scitranslmed.3002442
Zheng P, Liang C, Ren L, Zhu D, Feng Q, Chang W, Xu J (2018) Additional biomarkers beyond RAS that impact the efficacy of cetuximab plus chemotherapy in mCRC: a retrospective biomarker analysis. J Oncol 2018:5072987. https://doi.org/10.1155/2018/5072987
Funding
Supported by The National Natural Science Foundation of China (82072678, 82072653, 81372315); Shanghai Science and Technology Committee Project (19511121301, 17411951300); Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive (17DZ2252600); The National Key R&D Program of China (2017YFC0908200).
Author information
Authors and Affiliations
Contributions
(1) Conception/design: WH, YC, WC, LR, YW, JX; (2) Provision of study material or patients: WH, YC, WC, LR, YW, JX; (3) Collection and/or assembly of data: WH, YC, WC, LR, WT, PZ, QW; (4) Data analysis and interpretation: WH, YC, PZ, QW, TL, YL; (5) Manuscript writing: WH, YC, WC, LR; (6) Final approval of manuscript: WH, YC, WC, LR, WT, PZ, QW, TL, YL, YW, JX.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared no competing interests.
Ethical approval
Approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University.
Informed consent
Informed consents were acquired from all patients for the acquisition of clinical and pathological information and the use of surgical specimens.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, W., Chen, Y., Chang, W. et al. HER2 positivity as a biomarker for poor prognosis and unresponsiveness to anti-EGFR therapy in colorectal cancer. J Cancer Res Clin Oncol 148, 993–1002 (2022). https://doi.org/10.1007/s00432-021-03655-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03655-x