Abstract
Purpose
Although immune-checkpoint inhibitors have become a new therapeutic option for recurrent/metastatic non-small cell lung cancers (R/M-NSCLC), its clinical benefit in the real-world is still unclear.
Methods
We investigated 1181 Korean patients with programmed death-1 ligand 1 (PD-L1)-positive [tumor proportion score (TPS) ≥ 10% by the SP263 assay or ≥ 50% by the 22C3 assay] R/M-NSCLC treated with pembrolizumab or nivolumab after failure of platinum-based chemotherapy.
Results
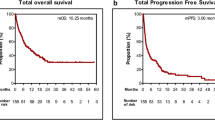
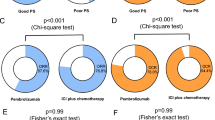
The median age was 67 years, 13% of patients had ECOG-PS ≥ 2, and 27% were never-smokers. Adenocarcinoma was predominant (61%) and 18.1% harbored an EGFR activating mutation or ALK rearrangement. Pembrolizumab and nivolumab were administered to 51.3% and 48.7, respectively, and 42% received them beyond the third-line chemotherapy. Objective response rate (ORR) was 28.6%. Pembrolizumab group showed numerically higher ORR (30.7%) than the nivolumab group (26.4%), but it was comparable with that of the nivolumab group having PD-L1 TPS ≥ 50% (32.4%). Median progression-free survival (PFS) and overall survival (OS) were 2.9 (95% CI 0–27.9) and 10.7 months (95% CI 0–28.2), respectively. In multivariable analysis, concordance of TPS ≥ 50% in both PD-L1 assays and the development of immune-related adverse events (irAEs) were two significant predictors of better ORR, PFS, and OS. EGFR mutation could also predict significantly worse OS outcomes.
Conclusion
The real-world benefit of later-line anti-PD1 antibodies was comparable to clinical trials in patients with R/M-NSCLC, although patients generally were more heavily pretreated and had poorer ECOG-PS. Concordantly high PD-L1 TPS ≥ 50% and development of irAE could independently predict better treatment outcomes, while EGFR mutation negatively affected OS.


Similar content being viewed by others
References
Ahn BC et al (2019) Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol 145:1613–1623. https://doi.org/10.1007/s00432-019-02899-y
Borghaei H et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Brahmer J et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. https://doi.org/10.1056/NEJMoa1504627
Buttner R et al (2017) Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol 35:3867–3876. https://doi.org/10.1200/jco.2017.74.7642
Das S, Johnson DB (2019) Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors . J Immunother Cancer 7:306. https://doi.org/10.1186/s40425-019-0805-8
Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Gandhi L et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Garassino MC et al (2018) Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 19:521–536. https://doi.org/10.1016/s1470-2045(18)30144-x
Garon EB et al (2019) Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 37:2518–2527. https://doi.org/10.1200/JCO.19.00934
Haratani K et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4:374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Hastings K et al (2019) EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer . Ann Oncol Off J Eur Soc Med Oncol 30:1311–1320. https://doi.org/10.1093/annonc/mdz141
Health Insurance Review and Assessment Service K (2019) Administrative rules for listed anti-cancer drugs (2020–81)
Herbst RS et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567. https://doi.org/10.1038/nature14011
Herbst RS et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet Lond Engl 387:1540–1550. https://doi.org/10.1016/s0140-6736(15)01281-7
Hirsch FR et al (2017) PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 12:208–222. https://doi.org/10.1016/j.jtho.2016.11.2228
Horn L et al (2017) Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 35:3924–3933. https://doi.org/10.1200/jco.2017.74.3062
Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC (2017) Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer—a meta-analysis . J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 12:403–407. https://doi.org/10.1016/j.jtho.2016.10.007
Mazieres J et al (2019) Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry . Ann Oncol Off J Eur Soc Med Oncol 30:1321–1328. https://doi.org/10.1093/annonc/mdz167
National Institutes of Health NCI, U.S. Department of Health and Human services (2017) Common terminology criteria for adverse events (CTCAE), Version 5.0
Ogata T, Satake H, Ogata M, Hatachi Y, Yasui H (2018) Hyperprogressive disease in the irradiation field after a single dose of nivolumab for gastric cancer: a case report . Case Rep Oncol 11:143–150. https://doi.org/10.1159/000487477
Reck M et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Rittmeyer A et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet Lond Engl 389:255–265. https://doi.org/10.1016/s0140-6736(16)32517-x
Rizvi NA et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Sci NY NY) 348:124–128. https://doi.org/10.1126/science.aaa1348
Roach C et al (2016) Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol 24:392–397. https://doi.org/10.1097/pai.0000000000000408
Rozenblum AB, Ilouze M, Dudnik E, Dvir A, Soussan-Gutman L, Geva S, Peled N (2017) Clinical impact of hybrid capture-based next-generation sequencing on changes in treatment decisions in lung cancer . J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 12:258–268. https://doi.org/10.1016/j.jtho.2016.10.021
Seifert L et al (2016) Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology 150:1659-1672.e1655. https://doi.org/10.1053/j.gastro.2016.02.070
Smit HJM et al (2020) Effects of checkpoint inhibitors in advanced non-small cell lung cancer at population level from the National Immunotherapy Registry. Lung Cancer Amst Neth 140:107–112. https://doi.org/10.1016/j.lungcan.2019.12.011
Suresh K et al (2019) Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy . J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 14:494–502. https://doi.org/10.1016/j.jtho.2018.11.016
Torlakovic E et al (2020) “Interchangeability” of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol 33:4–17. https://doi.org/10.1038/s41379-019-0327-4
Tournoy KG et al (2018) Does nivolumab for progressed metastatic lung cancer fulfill its promises? An efficacy and safety analysis in 20 general hospitals. Lung Cancer Amst Neth 115:49–55. https://doi.org/10.1016/j.lungcan.2017.11.008
Velcheti V, Patwardhan PD, Liu FX, Chen X, Cao X, Burke T (2018) Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PLoS ONE 13:e0206370–e0206370. https://doi.org/10.1371/journal.pone.0206370
Yoo SH, Keam B, Kim M, Kim TM, Kim DW, Heo DS (2018) Generalization and representativeness of phase III immune checkpoint blockade trials in non-small cell lung cancer. Thoracic cancer 9:736–744. https://doi.org/10.1111/1759-7714.12641
Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F (2020) Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 18:87. https://doi.org/10.1186/s12916-020-01549-2
Acknowledgements
Current multicenter research was initiated and supported by the collaboration from Health Insurance Review and Assessment Service of Korea (201812072D200) and by the Korean Cancer Study Group (KCSG LU19-05). And this work was supported by Konkuk University Medical Center Research Grant 2018. Lastly, we would like to thank Editage (www.editage.co.kr) for English language editing.
Author information
Authors and Affiliations
Contributions
LSK SMO gave a core assistance in data collection and developing a raw registry, which was thoroughly guided by JHP and JHK. GLR and HA made a final dataset, and made all statistical analyses for the study. JHP reviewed the literature, interpreted the data analysis, and mainly drafted the article. JHK initially inspired and motivated the concept of present study, who finally reviewed and confirmed the final version to be published. All co-authors listed equally contributed to enrollment of patients as expert medical oncologists or pulmonologists of lung cancer, and they all read and approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
No author has any financial disclosures to declare related to this study.
Research involving human participants
It was conducted in full accordance with the guidelines for the Good Clinical Practice and the 1964 Declaration of Helsinki.
Informed consent to participate
The study protocol was reviewed and approved by the institutional review board (of each participant center), and informed consents were achieved from all individual participants included in the study.
Informed consent to publication
All co-authors included in the study agreed with publication of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, J.H., You, G.L., Ahn, MJ. et al. Real-world outcomes of anti-PD1 antibodies in platinum-refractory, PD-L1-positive recurrent and/or metastatic non-small cell lung cancer, and its potential practical predictors: first report from Korean Cancer Study Group LU19-05. J Cancer Res Clin Oncol 147, 2459–2469 (2021). https://doi.org/10.1007/s00432-021-03527-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03527-4