Abstract
Purpose
The study was aimed to describe the characteristics of cancer patients admitted to the oncological ICU and to identify clinical features associated with outcomes.
Methods
This is a prospective study (January 2014 to December 2015) of 522 cancer patients consecutively admitted to the oncological ICU. Patients with a length of oncological ICU stay ≤ 1 day were excluded. Demographic and clinical variables were obtained at oncological ICU admission. The primary outcome of interest was hospital mortality. Logistic regression analysis was performed to identify independent risk factors for hospital mortality.
Results
The study cohort consisted of 492 (94.3%) patients with solid tumours and 30 patients (5.7%) with haematological malignancies. Advanced cancer was observed in 53.3%. Unplanned admission accounted for 25.3%. Hospital mortality rate was 13.0% (n = 68), and it was higher for patients with unplanned admission than those for electively admitted patients (35.6% vs. 5.4; p < 0.0001). Stage IV of cancer (OR 5.28; 95% CI 2.71–10.28; p < 0.0001), patients from the emergency department (OR 3.33; 95% CI 1.68–6.61; p = 0.001), unplanned admission (OR 7.99; 95% CI 4.45–14.33; p < 0.0001), non-malignancy-related admission (OR 5.80; 95% CI 3.26–10.32; p < 0.0001), sepsis (OR 4.81; 95% CI 2.28–10.16; p < 0.0001), chemotherapy-induced adverse event (OR 5.64; 95% CI 2.33–13.66; p < 0.0001), and invasive mechanical ventilation (OR 18.70; 95% CI 9.93–35.21; p < 0.0001) were independently associated with increased hospital mortality in multivariate logistic regression analysis.
Conclusions
ICU admission of cancer patients should be based on potential chance of recovering from the acute problem. Clinical predictor for mortality could support this purpose (UIN: researchregistry3484).
Similar content being viewed by others
Introduction
Numbers of patients living with cancer are increasing because of advances in the treatment of malignancies (Jemal et al. 2017). Cancer-related complications are common during the course of the disease (Torres et al. 2016). Accordingly, many of these patients require admission to the intensive care unit (ICU) for the management of underlying pathophysiological disorders such as postoperative status, respiratory failure, and sepsis (Schellongowski et al. 2016).
Organ support methods and sepsis management is the mainstay of treatment for critically ill patients with cancer. However, some malignancy-specific problems including oncological emergencies, organ dysfunction due to expansive or infiltrative cancer, chemotoxicity, radiotoxicity, tumour lysis syndrome, leukostasis, and hemophagocytic lymphohistiocytosis require a specialized therapy (Young and Simmons 2014; Lehmberg et al. 2015; Olcina and Giaccia 2016; Strauss et al. 2017). Thus, a close collaboration between the intensivist and oncologist is mandatory.
Mortality rates of critically ill cancer patients have decreased in the latest decades, because of advances in the management of malignancies and organ failures; however, some studies suggest that mortality of patients with cancer remains higher compared with that in patients without cancer (Taccone et al. 2009). Reasons of ICU admission, nature and number of organ failures, type of malignancy, and therapies before ICU admission may affect outcomes (Kostakou et al. 2014). According to methodological design and included patients, the ICU mortality rate in cancer patients range between 30 and 77% (Anisoglou et al. 2013; Mânica et al. 2013; Ostermann et al. 2013; Yoo et al. 2013; Aygencel et al. 2014). In addition, the ICU mortality rate for critically ill ventilated patients with cancer is greater than 45% (Almeida et al. 2014; Martos-Benítez et al. 2017). The study was aimed to describe the characteristics of cancer patients admitted to an oncological ICU (OICU) and to identify clinical features associated with outcomes.
Patients and methods
Design and setting
This was a prospective cohort study conducted in the OICU of the Institute of Oncology and Radiobiology (IOR) from January 2014 to December 2015. This is a 220-bed, university-affiliated, tertiary care referral centre for cancer patients in Havana, Cuba. The OICU has 12 beds and provides care for about 500 medical and surgical cancer patients per year. The current study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Scientific Council and the Ethics Committee for Scientific Research of the IOR (no. 54/02-11-2013). Written informed consent was obtained from all included patients.
Participants
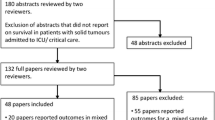
Over the study period, a total of 992 consecutive cancer patients were included independently of histology, tumour location, clinical stage, or reason for ICU admission. Patients with a length of ICU stay ≤ 1 day were excluded (Fig. 1); they may be divided in two subgroups: (1) patients undergoing intermediate-risk surgery (e.g., head and neck, major gynaecological, major urological) admitted to the ICU for early postoperative surveillance in accordance with institutional protocol (406 patients); and (2) patients who died within 24 h after ICU admission (63 patients). These patients were considered as unsalvageable, independently of any therapeutic effort, because of their severe pathophysiological disturbance; therefore, they can show basic features that distinguish them from general cancer patients admitted to the ICU. Thus, their exclusion reduced the risk of selection bias.
For those patients who were admitted more than once to the ICU during the same hospitalization, only the first data on ICU admission were analysed. To be admitted, the oncologist and the ICU physician generally were agreed that patients had a potential chance of recovering from the acute problem. In contrast, patients without any further oncology-specific treatment options were offered end-of-life care on the referring ward and not transferred to the ICU.
Data collection and outcomes
The following demographic and clinical data were obtained at ICU admission: age, sex, Age-Adjusted Charlson Comorbidity Index score with the exclusion of malignancy, primary tumour location, clinical stage of cancer, patient origin (hospital ward or emergency department), type of admission (elective admission or unplanned admission), nature of admission (malignancy-related or non-malignancy-related), Acute Physiology and Chronic Health Evaluation (APACHE) II score, transfusion of blood products within 15 days prior to ICU admission, sepsis, chemotherapy-induced adverse event as reason for admission in ICU, and requirement of invasive mechanical ventilation (MV).
Malignancy-related admission was defined as a cause directly related with tumoural tissue [e.g., primary surgical intervention (tumoural resection or tumoural occlusion/perforation), tumoural infection/haemorrhage, tumour lysis syndrome, paraneoplastic syndrome, and infiltrative or metastatic organ dysfunction]. Otherwise, the nature of admission was categorized as non-malignancy-related (e.g., infection/haemorrhage of non-tumoural tissue, surgical reintervention due to any complication of primary surgery, and adverse event to oncospecific therapy).
The primary outcome of interest was hospital mortality. Secondary outcomes were ICU mortality, length of ICU stay, ICU re-admission, and length of hospitalization.
Statistical analysis
Categorical variables are showed as count with percentage and numerical variables as median with 25th–75th interquartile range (IQR). Difference between groups was performed using Pearson’s Chi-square test (2) for categorical variables; because of lack of normality, the Mann–Whitney U test was used for numerical variables.
Multivariate logistic regression analysis was used to identify risk factors associated with hospital mortality. Because of lack of normality, APACHE II score was transformed by natural logarithm (Ln). Then, Ln(APACHE II) and Age-Adjusted Charlson Comorbidity Index were included as confounders, and a model for each clinical characteristic was built. Only those explanatory variables yielding p values ≤ 0.25 in the univariate analysis and obvious clinical implications were considered to enter in logistic regression models. Parsimony of the models was guaranteed. The Hosmer–Lemeshow test was used to check the goodness of fit. Discrimination capability was evaluated by determination of the area under the receiver-operating characteristic (ROC) curve. Results were summarized as odds ratio (OR) and respective 95% confidence interval (CI).
Statistical test with a two tailed p value ≤ 0.05 was considered as significant. Data were analysed using IBM® SPSS® Statistics 23.0 (IBM, Chicago, IL, USA).
Results
Characteristics of study population
A total of 522 patients were analysed (Fig. 1). The median age was 60.5 years (IQR 51.0–69.0 years). The median in the Age-Adjusted Charlson Comorbidity Index score was 3.0 points (IQR 2.0–5.0 points). The most common primary tumours were localized in gastrointestinal tract (35.2%), thorax (25.3%), and gynaecological tract (10.0%). Advanced cancer was observed in 53.3% of patients (stage III 29.7%; stage IV 23.6%). Four hundred and seventy-one patients (90.2%) arrived to ICU from hospital ward. Unplanned ICU admission accounted for 25.3% of patients (Table 1). The most common reasons for unplanned admission were acute respiratory failure (36.7%), infections (32.1%), cardiovascular disorders (19.3%), neurological disorders (6.4%), and emergency surgery (4.4%).
The median APACHE II score was 12.0 points (IQR 10.0–14.0 points), and the estimated probability of death was 11.7% (IQR 8.5–18.5%). Invasive ventilatory support was required for 15.5% of patients; the median ventilation time was 9.0 days (IQR 6.0–15.0 days). Eighty-one patients (15.5%) required vasopressors during their stay in ICU. The main characteristics of study population are depicted in Table 1.
Outcomes of cancer patients admitted to intensive care unit
The overall ICU mortality rate and overall hospital mortality rate was 10.2 and 13.0%, respectively. The median length of ICU and hospital stay was 3.0 and 9.0 days, respectively. Twenty-three patients were re-admitted to ICU during the same hospitalization. ICU mortality (p < 0.0001), length of ICU stay (p = 0.001), and hospital mortality (p < 0.0001) were higher for patients with unplanned admission than those for electively admitted patients. Table 2 summarizes the outcomes of studied participants.
In the univariate analysis, Age-Adjusted Charlson Comorbidity Index (p = 0.001), clinical stage of cancer (stage I 0.0% vs. stage II 20.8% vs. stage III 20.8% vs. stage IV 58.5%; p < 0.0001), patients origin (hospital ward 75.5% vs. emergency department 24.5%; p < 0.0001), type of admission (elective 28.3% vs. unplanned 71,7%; p < 0.0001), nature of admission (malignancy-related 49.1% vs. non-malignancy-related 50.9%; p < 0.0001), sepsis (73.6 vs. 26.45%; p < 0.0001), chemotherapy-induced adverse event (79.2 vs. 20.8%; p < 0.0001), invasive mechanical ventilation (66.0 vs. 34.0%; p < 0.0001), and APACHE II score (p = 0.001) were related with ICU mortality.
With regard to hospital mortality, Table 1 shows that higher Age-Adjusted Charlson Comorbidity Index score, primary tumour location, higher clinical stage of cancer, patients admitted from emergency department, unplanned admission, non-malignancy-related admission, sepsis, chemotherapy-induced adverse event, invasive mechanical ventilation, and higher APACHE II score were related to a higher hospital mortality rate in univariate analysis.
In multivariate logistic regression analysis, after adjust for confounders, stage IV of cancer, patients admitted from emergency department, unplanned admission, non-malignancy-related admission, sepsis, chemotherapy-induced adverse event, and invasive mechanical ventilation were independently associated with increased risk of hospital mortality (Table 3).
Discussion
This prospective study showed an overall ICU and hospital mortality rate of 10.2 and 13.0%, respectively. Mortality rates were higher in the previous studies (Anisoglou et al. 2013; Mânica et al. 2013; Ostermann et al. 2013; Yoo et al. 2013; Aygencel et al. 2014). Differences in the composition of the studied participants could explain these differences; for example, elective postoperative patients prevailed in our study. In addition, MV and vasopressors were only required for 15.5% of patients. In this regard, characteristics of cancer patients admitted to ICU vary around the world. Results of studies analyzing a wide cohort of patients are similar to those found by us, with a VM and vasopressors rate of 18.0–28.4 and 10.8–23.3%, respectively (Soares et al. 2010; Xing et al. 2015; Fisher et al. 2016; Shrime et al. 2016). However, ICU and hospital mortality rate was 28.8 and 35.6%, respectively, when unplanned patients were analysed. These mortality rates are comparable to those reported in international cohorts (Bos et al. 2012; Aygencel et al. 2014; Fisher et al. 2016).
The major findings of this study were that stage IV of cancer, patients admitted from the emergency department, unplanned admission, non-malignancy-related admission, sepsis, chemotherapy-induced adverse event, and invasive mechanical ventilation were independent clinical risk factors for hospital mortality in multivariate analysis.
Advanced cancer has been associated with higher mortality in the previous studies (Fisher et al. 2016; Xia and Wang 2016). Such relationship could be explained by two ways: (1) because of infiltrative/ metastatic organ dysfunction; (2) increasing the risk of other mortality-related factors such as acute respiratory failure (ARF) and sepsis. In fact, both severe ARF requiring MV and sepsis were factors independently associated with mortality in our study. For ventilated cancer patients, ICU mortality rates and hospital mortality rates have been reported greater than 50% (Almeida et al. 2014; Martos-Benítez et al. 2017) and 65% (Azevedo et al. 2014; Martos-Benítez et al. 2017), respectively. Barotrauma, oxygen toxicity, hemodynamic compromise, ventilator-induced lung injury, ventilator-associated pneumonia, as well as local and systemic effects of tumour explain higher mortality associated with MV (Slutsky and Ranieri 2013; Blot et al. 2014; Serpa et al. 2014; Spieth et al. 2014). On the other hand, sepsis is a major risk factor for mortality among critically ill cancer patients (Schellongowski et al. 2016). Recent data suggest that active disease, haematological malignancies, compromised performance status, presence of 3–4 systemic inflammatory response syndrome criteria, the number of acute organ dysfunctions, and polymicrobial infections are associated with increased mortality in these subjects (Rosolem et al. 2012; Torres et al. 2015).
Patients with cancer are frequently encountered in the emergency department due to clinical conditions requiring ICU admission such as infection, acute respiratory failure, need of emergency surgery, haemorrhage, and complications of chemotherapy (Young and Simmons 2014; Cornillon et al. 2016; Lash et al. 2017). Recently, García-Gigorro et al. found that ICU complications such as acute renal failure and acute respiratory distress syndrome were the variables with the greatest impact on hospital mortality among patients admitted to ICU from the emergency department (García-Gigorro et al. 2017).
In cancer patients with unplanned admission to ICU, the hospital mortality rate ranges between 30 and 55% (Bos et al. 2012; Aygencel et al. 2014; Fisher et al. 2016). In addition, mortality rate is almost twice higher in medical cancer patients (40.6%) than in medical patients without cancer (23.7%) as recently reported Bos et al. (Bos et al. 2012). The presence of metastasis, APACHE II score, a Glasgow Coma Scale < 7 on admission to ICU, sepsis/septic shock, and vasopressor requirement during ICU stay are the main mortality-explicative variables (Aygencel et al. 2014; Fisher et al. 2016).
The nature of admission has not been analysed in the previous studies, so it is an important contribution of this study with significant clinical implication because of their impact on prediction of mortality. We considered malignancy-related admission when tumoural tissue was directly implicated as cause of ICU admission. Otherwise, the nature of admission was defined as non-malignancy-related. This definition should be used in future studies to be compared with our results.
A recent systematic review suggests that adverse drug events requiring ICU admission are frequents (Jolivot et al. 2014). Oncospecific-drug therapy is associated with several adverse events leading clinical decline, impairment of quality of life, and death (Davis 2015). In fact, disorders such as neutropenia, mucositis, pneumonitis, and encephalopathy are commonly related with drugs toxicity and mortality in cancer patients admitted to ICU (Legrand et al. 2012; Yoo et al. 2013; Iacovelli et al. 2014).
Length of ICU stay and time of hospitalization was lower than those reported in specific subgroups of critically ill cancer patients (e.g., cancer patients with sepsis, lung cancer patients, and cancer patients with acute respiratory failure) (Mânica et al. 2013; Ferreira et al. 2015; Torres et al. 2015). However, length of stay was comparable with those described in studies analyzing a wide cohort of critically ill patients with cancer (Soares et al. 2010; Bos et al. 2012; Xing et al. 2015; Fisher et al. 2016), including a large prospective study carried out in several ICU of Europe (Taccone et al. 2009).
The present study has several shortcomings. First, our study was conducted at a specialized ICU of single institution confined to oncological patients. Therefore, the result of this study may not be generalized to other general medical centres. Second, there were no patients who had leukaemia, and haematological patients represented a small number of participants. Finally, we have evaluated a relatively large number of patients for a single centre study; however, it could be considered as a limitation.
Conclusions
Clinical predictors of hospital mortality for cancer patients admitted to the ICU are advanced cancer, patients admitted from the emergency department, unplanned admission, non-malignancy-related admission, sepsis, chemotherapy-induced adverse event, and invasive mechanical ventilation. The decision to admit critically ill patients with cancer to the ICU should be based on the probability of surviving the acute illness and not on malignance by itself. Consequently, a general reluctance to admit these patients to the ICU is not justified. Palliative care is a justified alternative for patients with severe pathophysiological disturbances whom ICU admission is considered as futile; but, critically ill patients with cancer should always receive a specialized care. Prospective studies examining the impact of these and others clinical prognostic factors on outcomes are warranted.
References
Almeida IC, Soares M, Bozza FA, Shinotsuka CR, Bujokas R, Souza-Dantaset VC et al (2014) The impact of acute brain dysfunction in the outcomes of mechanically ventilated cancer patients. PLoS One 9:e85332
Anisoglou S, Asteriou C, Barbetakis N, Kakolyris S, Anastasiadou G, Pnevmatikos I (2013) Outcome of lung cancer patients admitted to the intensive care unit with acute respiratory failure. Hippokratia 17:60–63
Aygencel G, Turkoglu M, Turkoz G, Benekli M (2014) Prognostic factors in critically ill cancer patients admitted to the intensive care unit. J Crit Care 29:618–626
Azevedo LC, Caruso P, Silva UV, Torelly AP, Silva E, Rezende E et al (2014) Outcomes for patients with cancer admitted to the ICU requiring ventilatory support: results from a prospective multicenter study. Chest 146:257–266
Blot SI, Poelaert J, Kollef M (2014) How to avoid microaspiration? A key element for the prevention of ventilator-associated pneumonia in intubated ICU patients. BMC Infect Dis 14:119
Bos MM, de Keizer NF, Meynaar IA, Bakhshi-Raiez F, de Jonge E (2012) Outcomes of cancer patients after unplanned admission to general intensive care units. Acta Oncol 51:897–905
Cornillon P, Loiseau S, Aublet-Cuvelier B, Guastella V (2016) Reasons for transferral to emergency departments of terminally ill patients—a French descriptive and retrospective study. BMC Palliative Care 15:87
Davis C (2015) Drugs, cancer and end-of-life care: a case study of pharmaceuticalization? Social Sci Med 131:207214
Ferreira JC, Medeiros P Jr, Rego FM, Caruso P (2015) Risk factors for noninvasive ventilation failure in cancer patients in the intensive care unit: a retrospective cohort study. J Crit Care 30:1003–1007
Fisher R, Dangoisse C, Crichton S, Crichton S, Whiteley C, Camporota L et al (2016) Short-term and medium-term survival of critically ill patients with solid tumours admitted to the intensive care unit: a retrospective analysis. BMJ Open 6:e011363
García-Gigorro R, Dominguez Aguado H, Barea Mendoza JA, Viejo Moreno R, Sánchez Izquierdo JA, Montejo-González JC (2017) Short- and long-term prognosis of critically-ill patients referred to the ICU from the Emergency Department of a tertiary hospital. Med Clin (Barc) 148:197–203
Iacovelli R, Pietrantonio F, Palazzo A, Maggi C, Ricchini F, de Braud F et al (2014) Incidence and relative risk of grade 3 and 4 diarrhoea in patients treated with capecitabine or 5- fluorouracil: a meta-analysis of published trials. Br J Clin Pharmacol 78:1228–1237
Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB et al (2017) Annual report to the nation on the status of cancer, 1975–2014, featuring survival. JNCI 109:djx030
Jolivot PA, Hindlet P, Pichereau C, Fernandez C, Maury E, Guidet B et al (2014) A systematic review of adult admissions to ICUs related to adverse drug events. Crit Care 18:643
Kostakou E, Rovina N, Kyriakopoulou M, Koulouris NG, Koutsoukou A (2014) Critically ill cancer patient in intensive care unit: issues that arise. J Crit Care 29:817–822
Lash RS, Bell JF, Reed SC, Poghosyan H, Rodgers J, Kim KK et al (2017) A systematic review of emergency department use among cancer patients. Cancer Nurs 40:135–144
Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A et al (2012) Survival in neutropenic patientswith severe sepsis or septic shock. Crit Care Med 40:43–49
Lehmberg K, Nichols KE, Henter JI, Girschikofsky M, Greenwood T, Jordan M et al (2015) Consensus recommendations for the diagnosis and management of hemophagocytic lymphohistiocytosis associated with malignancies. Haematologica 100:997–1004
Mânica A, Basso M, Rossato D (2013) Outcomes for patients with lung cancer admitted to intensive care units. Rev Bras Ter Intensiva 25:12–16
Martos-Benítez FD, Gutiérrez-Noyola A, Badal M, Dietrich NA (2017) Risk factors and outcomes of severe acute respiratory failure requiring invasive mechanical ventilation in cancer patients: a retrospective cohort study. Med Intensiva. https://doi.org/10.1016/j.medin.2017.08.004
Olcina MM, Giaccia AJ (2016) Reducing radiation-induced gastrointestinal toxicity—the role of the PHD/HIF axis. J Clin Invest 126:3708–3715
Ostermann M, Raimundo M, Williams A, Whiteley C, Beale R (2013) Retrospective analysis of outcome of women with breast or gynaecological cancer in the intensive care unit. J R Soc Med Sh Rep 4:2
Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV et al (2012) Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care 27:301–307
Schellongowski P, Sperr WR, Wohlfarth P, Knoebl P, Rabitsch W, Watzke HH et al (2016) Critically ill patients with cancer: chances and limitations of intensive care medicine—a narrative review. ESMO Open 1:e000018
Serpa A, Filho RR, Rocha LL, Schultz MJ (2014) Recent advances in mechanical ventilation in patients without acute respiratory distress syndrome. F1000Prime Rep 6:115
Shrime MG, Ferket BS, Scott DJ, Lee J, Barragan-Bradford D, Pollard T et al (2016) Time-limited trials of intensive care for critically ill patients with cancer: how long is long enough? JAMA Oncol 2:76–83
Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369:2126–2136
Soares M, Silva UVA, Teles JMM, Silva E, Caruso P, Lobo SM et al (2010) Validation of four prognostic scores in patients with cancer admitted to Brazilian intensive care units: results from a prospective multicenter study. Intensive Care Med 36:1188–1195
Spieth PM, Koch T, de Abreu MG (2014) Approaches to ventilation in intensive care. Dtsch Arztebl Int 111:714–720
Strauss PZ, Hamlin SK, Dang J (2017) Tumor Lysis Syndrome: a unique solute disturbance. Nurs Clin North Am 52:309–320
Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent JL (2009) Characteristics and outcomes of cancer patients in European ICUs. Crit Care 13:R15
Torres VB, Azevedo LC, Silva UV, Caruso P, Torelly AP, Silva E et al (2015) Sepsis-associated outcomes in critically ill patients with malignancies. Ann Am Thorac Soc 12:1185–1192
Torres VB, Vassalo J, Silva UV, Caruso P, Torelly AP, Silva E et al (2016) Outcomes in critically ill patients with cancer-related complications. PLoS One 11:e0164537
Xia R, Wang D (2016) Intensive care unit prognostic factors in critically ill patients with advanced solid tumors: a 3-year retrospective study. BMC Cancer 16:188
Xing X, Gao Y, Wang H, Huang C, Qu S, Zhang H et al (2015) Performance of three prognostic models in patients with cancer in need of intensive care in a medical center in china. PLoS One 10:e0131329
Yoo H, Young G, Jeong BH, Yeon S, Pyo M, Jung O et al (2013) Etiologies, diagnostic strategies, and outcomes of diffuse pulmonary infiltrates causing acute respiratory failure in cancer patients: a retrospective observational study. Crit Care 17:R150
Young JS, Simmons JW (2014) Chemotherapeutic medications and their emergent complications. Emerg Med Clin North Am 32:563–578
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Martos-Benítez, F.D., Soto-García, A. & Gutiérrez-Noyola, A. Clinical characteristics and outcomes of cancer patients requiring intensive care unit admission: a prospective study. J Cancer Res Clin Oncol 144, 717–723 (2018). https://doi.org/10.1007/s00432-018-2581-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2581-0