Abstract
Purpose
The clinical features of patients with advanced non-small cell lung cancer (NSCLC) and interstitial lung disease (ILD) have not fully been elucidated. This study aimed to investigate the clinical features of these patients, particularly with idiopathic pulmonary fibrosis (IPF).
Methods
Data on 218 patients with pathologically confirmed diagnoses of NSCLC who had been treated with chemotherapy and/or molecular targeted therapy were retrospectively analyzed for progression-free survival (PFS), overall survival (OS), responses to first-line therapy, and incidence of acute exacerbations (AEs).
Results
Fifty-three of the 218 patients were diagnosed with ILD, and 34 of them with IPF. The frequency of epidermal growth factor receptor (EGFR) mutation was significantly lower in ILD and IPF patients than in non-ILD patients (2 or 0 vs. 32 %, respectively). Median PFS and OS were significantly shorter in both ILD and IPF patients than in non-ILD patients (118, 92, and 196 days for PFS, and 267, 223, and 539 days for OS, respectively). Multivariate analysis showed that poor performance status, absence of EGFR mutation, and presence of IPF were poor prognostic factors for PFS and OS. Disease control rate (DCR) was significantly lower in ILD and IPF patients than in non-ILD patients regardless of the presence of EGFR mutation (67 or 53 vs. 85 %, respectively). The incidence of AEs of ILD was significantly higher during chemotherapy with docetaxel-containing regimens (seven of 38; 18.4 %).
Conclusions
Both IPF and ILD were associated with lower EGFR positivity, lower DCR, and shorter PFS and OS in advanced NSCLC patients.
Similar content being viewed by others
Introduction
Interstitial lung diseases (ILDs), also called interstitial lung abnormalities (ILA) or interstitial pneumonia (IP), are characterized by diffuse pulmonary interstitial abnormalities that often lead to fibrosis (Borchers et al. 2011). Recent evidence has shown that preexisting ILD is associated with shorter survival in patients with advanced non-small cell lung cancer (NSCLC) (Kinoshita et al. 2012; Nishino et al. 2015). In a study of NSCLC patients receiving chemotherapy, 22 of whom had idiopathic interstitial pneumonia (IIP), IIP was found to be a significantly unfavorable factor for progression-free survival (PFS) (95.0 vs. 199.5 days) and overall survival (OS) (163.0 vs. 400.0 days) (Kinoshita et al. 2012). In another study, NSCLC patients with high ILA scores according to computed tomography (CT) findings had shorter OS (n = 17, 7.2 months) than those without ILA (n = 103, 14.8 months) (Nishino et al. 2015).
Idiopathic pulmonary fibrosis (IPF), the commonest type of ILD (Borchers et al. 2011), is defined as a specific form of chronic, progressive fibrosing IP of unknown cause, occurring primarily in older adults, limited to the lungs, and associated with the histopathologic and/or radiologic patterns of usual interstitial pneumonia (UIP) (Raghu et al. 2011). IPF was recently reported to be a poor prognostic factor in patients with surgically treated NSCLC (Goto et al. 2014; Lee et al. 2014). Only one study has focused on the efficacy of chemotherapy in advanced NSCLC with IPF (Watanabe et al. 2013). The authors reported that 21 patients with IPF had an overall response rate (RR), PFS, and OS of 42.9 %, 5.4, and 11.4 months, respectively (Watanabe et al. 2013). No published studies have compared the survival of IPF and non-ILD patients with advanced NSCLC.
Because there is limited clinical information regarding advanced NSCLC patients with IPF, we retrospectively compared the efficacy of chemotherapy and survival in NSCLC patients with ILD or IPF with that in patients without ILD. In addition, we investigated the incidence, risk factors, and outcome of acute exacerbations (AEs) after receiving chemotherapy.
Materials and methods
Patients
This study was approved by the Institutional Review Board of Kagawa University. Patients with pathologically confirmed advanced (stage IIIB or IV) NSCLC who presented to the Department of Internal Medicine, Kagawa University Hospital, between January 2007 and July 2015 were retrospectively identified, and relevant clinical and laboratory data were collected from their medical records. Patients who (1) had received definitive thoracic irradiation (usually more than 60 Gy), (2) had another concomitant active malignancy, or (3) had received only best supportive care were excluded, whereas those treated with chemotherapy and/or molecular targeted therapy such as tyrosine kinase inhibitors (TKIs) for epidermal growth factor receptor (EGFR) were included.
Classification of ILD and diagnosis of IPF and AE
The classification of ILD was made in accordance with the ATS/ERS/Japanese Respiratory Society/Latin American Thoracic Association Statement (Raghu et al. 2011). UIP pattern was defined as having all the following four features: (1) subpleural, basal predominance; (2) reticular abnormality; (3) honeycombing with or without traction bronchiectasis; and (4) absence of features listed as inconsistent with UIP pattern (Raghu et al. 2011). If honeycombing was absent, but other features met the criteria for UIP, the case was classified as possible UIP pattern (Raghu et al. 2011).
The diagnosis of IPF was based on the following criteria: (1) UIP pattern on high-resolution computed tomography and (2) exclusion of other known causes of interstitial lung diseases (Raghu et al. 2011). No patients in this study underwent surgical lung biopsy to diagnose IPF.
According to the sequential reading method, a board-certificated pulmonologist (N.K.) and a board-certificated radiologist (M.M.) independently reviewed all subjects’ CT scans and classified them into four categories: UIP pattern, possible UIP pattern, inconsistent with UIP pattern, and without ILD. Accordance of their classifications was considered a definite diagnosis. When their classifications differed, two other board-certificated pulmonologists (T.T. and A.T.) carefully reviewed the scans independently; the diagnosis was considered definite if the classifications of three of the four readers were in accord. When two readers agreed on one classification and the other two another, the case was discussed until all four readers agreed on a diagnosis (this procedure was required in two cases).
Diagnoses of AEs of ILD were made in accordance with criteria detailed in previous studies as follows: (1) worsening of dyspnea within 30 days; (2) new radiologic bilateral ground-glass abnormality and/or consolidation superimposed on a background of reticular shadows or honeycombing; (3) no evidence of pulmonary infection; and (4) exclusion of alternative causes, including left heart failure, pulmonary embolism, and acute lung injury of identifiable cause (Collard et al. 2007; Kinoshita et al. 2012).
Statistical analysis
PFS was defined as the time between the start of chemotherapy or molecular targeted therapy and diagnosis of disease progression or death. OS was defined as the time between the date of diagnosis and date of death from any cause. PFS and OS curves were constructed by the Kaplan–Meier method, and differences in survivals compared using the log-rank test. Fisher’s exact test and Student’s t test were used to analyze patient characteristics and the significance of the association of AE with ILD or IPF. Laboratory and pulmonary function data are presented as mean ± SD. All statistical analyses were performed using Ekuseru-Toukei 2015 (Social Survey Research Information, Tokyo, Japan).
Results
Patient selection
In all, 285 patients with pathologically confirmed advanced (stage IIIB or IV) NSCLC were identified for this study, 29 of whom had received definitive thoracic irradiation, four had another concomitant active malignancy (malignant lymphoma, renal, gastric, or colon cancer), and 34 had received only best supportive care. Thus, 218 patients were included in this study. Samples were obtained by transbronchial biopsy (131 cases), percutaneous biopsy (71 cases including 31 pleural effusions), surgical resection (9 cases), and others such as biopsy at different departments (7 cases).
Classification of ILD and diagnosis of IPF
Relevant characteristics of patients treated with chemotherapy and/or molecular targeted therapy are shown in Table 1. ILD was identified in 53/218 patients (24.3 %): 35 were diagnosed as having UIP, 15 possible UIP, and three inconsistent with UIP. One patient had dermatomyositis-related UIP pattern, and the remaining 34 with UIP pattern were diagnosed as having IPF (15.6 % of 218 patients).
Patients with ILD were significantly older than those without ILD and more often male and smokers (Table 1). The frequency of adenocarcinoma was lower, and that of squamous cell carcinoma was higher in patients with ILD than in those without it (40 vs. 78 % for adenocarcinoma, and 36 and 13 % for squamous cell carcinoma, respectively). EGFR mutation was detected in 53/112 non-ILD patients (32 %) and in only one of 53 ILD patients (2 %). These differences were more extreme in patients with IPF, all of whom were male smokers. Adenocarcinoma and squamous cell carcinoma histology each comprised 35 % of cases, and no EGFR mutations were detected. Average of percent vital capacity (%VC) is significantly lower in IPF than in non-ILD patients (76.4 % vs 89.1 %, respectively, P = 0.0115, data not shown).
Responses to chemotherapy and/or molecular targeted therapy
First-line therapy was cytotoxic chemotherapy in 179/218 patients and EGFR-TKI in the remaining 39 (Table 2). Fewer than three cycles of first-line chemotherapy were received by 72 and 82 % of ILD and IPF patients, respectively, both percentages being significantly higher than for non-ILD patients (37 %, P < 0.0001). RR evaluated by RECIST was 55, 37, and 31 % for non-ILD, ILD, and IPF patients, respectively. Disease control rate (DCR) was 87, 71, and 53 % in non-ILD, ILD, and IPF patients, respectively. RR and DCR were significantly lower in ILD and IPF patients than in non-ILD patients. When patients with EGFR mutation were excluded (n = 54), RR was 44, 35, and 31 % for non-ILD, ILD, and IPF patients, respectively. DCR was 85, 67, and 53 % for non-ILD, ILD, and IPF patients, respectively. Although RR was not significantly different in subjects with EGFR wild type (WT), the DCR was significantly lower in ILD and IPF than in non-ILD patients.
Shorter PFS and OS in patients with ILD or IPF
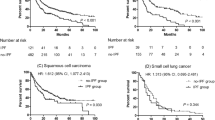
Kaplan–Meier survival curves for NSCLC patients who received chemotherapy and/or molecular targeted therapy showed a significantly shorter median PFS (Fig. 1a, 118 vs. 196 days, P = 0.0007) and OS (Fig. 1b, 267 vs 539 days, P = 0.001) in ILD than in non-ILD patients. In patients with IPF, median PFS (92 days, P < 0.0001) and OS (223 days, P = 0.0002) were even shorter (Fig. 2).
Univariate analysis by the log-rank test identified that male sex, smoking history, poor performance status (PS), non-adenocarcinoma histology, and EGFR-WT were associated with poor PFS and/or OS (Table 3). Multivariate analysis by Cox proportional hazards model showed that poor PS, absence of EGFR mutation, and presence of IPF were identified as poor prognostic factors for PFS and OS.
Because EGFR mutation status is associated with survival and differs between non-ILD and ILD/IPF patients, PFS and OS were assessed in these patients. Interestingly, PFS and OS were still shorter in ILD and IPF patients with EGFR-WT. Median PFS was 180, 118, and 92 days for non-ILD, ILD, and IPF patients, respectively (P = 0.0301 for non-ILD vs. ILD and P = 0.0018 for non-ILD vs. IPF). Median OS was 458, 258, and 223 days for non-ILD, ILD, and IPF patients, respectively (P = 0.0202 for non-ILD vs. ILD and P = 0.0126 for non-ILD vs. IPF).
Factors associated with AEs of ILD
AEs were observed in 10/53 patients (18.9 %) with ILD (Table 5). Limited to patients with IPF, AEs developed in 6/34 patients (17.6 %). No factors associated with the occurrence of AE were identified. However, the incidence of AEs differed between chemotherapeutic regimens (Table 4). The number of regimen types received by each patient was counted as shown by the following example: carboplatin plus pemetrexed plus bevacizumab followed by pemetrexed plus bevacizumab was classified as one regimen type. The same regimen administered to two patients was counted as two regimens. In all, 96 regimens were administered to 53 patients with ILD. When they contained docetaxel, the incidence of AEs was 18.4 % (7/38 regimens), which is significantly higher than for regimens without docetaxel (5.2 %, P = 0.0472). In IPF patients, docetaxel-containing regimens resulted in a trend toward higher incidence of AEs (5/24 patients; 20.8 %), whereas only 1/29 regimens (3.4 %) without docetaxel led to AEs (P = 0.0802); this difference is not statistically significant. No ILD patients received EGFR-TKI.
Median PFS in patients with and without AE was 118 versus 124 days (P = 0.4244), and median OS was 142 versus 284 days (P = 0.1099). Although these differences are not statistically significant, 6/10 patients died quickly after the onset of their AEs (Table 6). All patients who developed AEs were treated with corticosteroids, including methylprednisolone pulse therapy, and received no further chemotherapy.
The reasons for discontinuation of first-line chemotherapy within three cycles were investigated (Table 7). Interestingly, discontinuation because of adverse events occurred significantly more often in ILD and IPF than in non-ILD patients (42, 46, and 21 %, respectively). Importantly, 6/16 adverse events that resulted in discontinuation of chemotherapy were AEs in ILD patients. In IPF patients, AEs comprise 6/13 adverse events.
Discussion
In the present study, we demonstrated that (1) characteristics of ILD/IPF patients differed significantly from those of non-ILD patients; in particular, no IPF patients had EGFR mutation-positive tumors; (2) the presence of either IPF or ILD was associated with shorter PFS and OS in patients with advanced NSCLC who received chemotherapy; (3) DCR was significantly lower in ILD and IPF patients than in non-ILD patients, even those with EGFR-WT; and (4) AEs of ILD occurred more frequently after docetaxel-containing regimens than after other regimens.
The present study clearly demonstrated several differences in the characteristics of non-ILD and ILD/IPF patients. Most ILD and all 34 IPF patients were male smokers and had EGFR-WT. It is particularly interesting that no IPF patients had EGFR mutation-positive cancers, which has been shown for the first time although a previous study has reported a correlation between preexisting ILD and EGFR-WT adenocarcinoma (Fujimoto et al. 2013). In that study, only one of 31 ILD patients with lung adenocarcinoma had an EGFR mutation; this patient had a non-UIP radiographic pattern (Fujimoto et al. 2013). The current study extended this finding in that our one ILD patient with EGFR mutation also had a radiologic pattern inconsistent with UIP pattern. The frequency of squamous cell carcinoma was higher in IPF than in non-ILD patients (35 vs. 13 %). In ILD patients, most tumors reportedly develop in the area affected by ILD (Fujimoto et al. 2013; Kanaji et al. 2015). The current study provides evidence that carcinogenesis in IPF differs from that in non-ILD patients in that it is not associated with EGFR mutation.
Consistent with previous studies (Borchers et al. 2011; Watanabe et al. 2013), IPF was the commonest type of ILD (34/53 patients; 64 %). Even in patients with EGFR-WT, PFS and OS were significantly shorter in IPF than in non-ILD patients. These findings indicate that the presence of IPF predicts an extremely poor prognosis in patients with advanced NSCLC. Lower %VC (<80 %) was also associated with shorter survivals (120 vs 196 days for PFS, P = 0.0043, and 266 vs 538 days for OS, P = 0.0023, respectively, data not shown). Shorter survival in lower %VC has been reported in patients with NSCLC and ILD who underwent surgical resection (Sato et al. 2015).
Another important finding of the current study is regarding response to chemotherapy/molecular targeted therapy. RR and DCR in ILD/IPF patients were lower than that in non-ILD patients. EGFR mutation was not identified in any of the 34 IPF patients, whereas 32 % of non-ILD patients harbored EGFR mutations. Because the response to EGFR-TKI is much better in tumors with EGFR mutation than in EGFR-WT, the lower RR in IPF patients may be associated with the absence of EGFR mutations. Indeed, the difference in RR was not significant for EGFR-WT patients; interestingly, however, a difference in DCR was observed, even in patients without EGFR mutations.
There are several possible mechanisms that could explain the lower DCR and shorter survival of ILD/IPF patients. First, ILD/IPF patients may experience adverse events more frequently. Indeed, adverse events, including AEs, and PD were the main reasons for discontinuing first-line therapy within three cycles in patients with ILD/IPF, suggesting that AEs of ILD/IPF may have made an important contribution to the lower DCR and shorter survival. Consistent with this, coexisting ILD is reportedly associated with a high risk of developing chemotherapy-induced ILD (Sakurada et al. 2015). Chemotherapeutic strategies that do not induce AEs or deterioration of ILD are needed.
Second, an altered drug delivery system may lead to the lower response and shorter survival of ILD/IPF patients. Histological features of the UIP pattern include marked pulmonary fibrosis and architectural distortion (Raghu et al. 2011). Alveolar epithelial cell damage, dysregulation of fibroblasts, and vascular injury and aberrant angiogenesis related to vascular remodeling are key elements of lung fibrosis (Selman and Pardo 2006; Wémeau-Stervinou et al. 2012). Destruction of normal vasculature would logically reduce the local delivery of chemotherapeutic agents to a tumor.
Third, acquired drug resistance may result in lower DCR and shorter survival in ILD/IPF patients. In this regard, transforming growth factor (TGF)-β may have a role. Many cytokines and growth factors, including TGF-β, are reportedly associated with the development of ILD/IPF (Das et al. 2014; Kanaji et al. 2014). TGF-β concentrations are higher in bronchoalveolar lavage fluid obtained from IPF than from control patients (Khalil et al. 2001). TGF-β is reportedly associated with chemoresistance in colon cancer (Li et al. 2015). Additionally, TGF-β is a major regulator of epithelial–mesenchymal transition in the alveolar epithelia (Kasai et al. 2005; Miyazono 2009). In epithelial–mesenchymal transition, cancer cells seemingly acquire chemoresistance in lung and other types of cancers (Jiang et al. 2015; Li et al. 2014; Wang et al. 2015). Thus, increased TGF-β in the lung’s microenvironment may be a causative factor for lower DCR and shorter survival in NSCLC patients with ILD/IPF.
Regarding AEs of ILD, several studies have reported docetaxel-induced AE (Tamiya et al. 2012; Watanabe et al. 2015). In 35 patients with NSCLC and IP treated with docetaxel monotherapy, the incidence of AEs of IP was 14.3 % (5/35 patients). Three of the five patients who developed AEs of IP died (Watanabe et al. 2015). In another study, deterioration of ILD was observed in 7/27 patients (25.9 %) with NSCLC and preexisting ILD (Tamiya et al. 2012). In contrast, 6/309 patients (1.9 %) without preexisting ILD reportedly developed it after receiving docetaxel (Tamiya et al. 2012). In the current study, there was also a higher incidence of AEs of ILD/IPF after docetaxel-containing chemotherapy. Of note, AEs also occurred in 3/15 patients with radiographic possible UIP pattern (20 %). Several studies have reported that possible UIP pattern often corresponds with pathologically confirmed UIP (Sumikawa et al. 2014; Raghu et al. 2014). Radiographic possible UIP pattern should be considered clinically similar to UIP when choosing treatment strategies for patients with advanced NSCLC. Administration schedule also affects the incidence of AEs (higher incidence in a weekly than in triweekly administration) (Chen et al. 2006). Anti-microtubule activity by taxanes itself seems not to be related in the development of AE because the incidence of AE in paclitaxel is different from that in docetaxel. Thus, factors other than anticancer activity might be associated with docetaxel-related AE although an actual mechanism has never been reported.
No optimal chemotherapeutic regimen for patients with advanced NSCLC and underlying ILD has been established (Watanabe et al. 2015). A combination of carboplatin and weekly paclitaxel may be a treatment option for NSCLC with ILD (Minegishi et al. 2011). Eighteen patients with ILD, including six with IPF, treated with carboplatin and paclitaxel achieved PFS and median survival time of 5.3 and 10.6 months, respectively (Minegishi et al. 2011). Only one patient (5.6 %) with IPF developed an AE (after four cycles of chemotherapy). However, in another study 4/15 patients with NSCLC and ILD who received carboplatin and paclitaxel developed grade 3 or higher pneumonitis (27 %) (Shukuya et al. 2010); this high incidence cannot be ignored. In a phase III trial, administration of albumin-bound paclitaxel (nab-paclitaxel) as first-line treatment in patients with advanced NSCLC without ILD was effective and resulted in a higher RR than conventional solvent-based paclitaxel (Socinski et al. 2012). Currently, several clinical trials are assessing a combination of carboplatin and nab-paclitaxel for advanced NSCLC with ILD.
Nintedanib is a multiple tyrosine kinase inhibitor, and INPULSIS-2 trial demonstrated that nintedanib reduced the time to first AE compared with placebo (Richeldi et al. 2014). In addition, a combination of docetaxel and nintedanib showed a benefit on OS over docetaxel monotherapy as second-line treatment (Reck et al. 2014). The addition of nintedanib to cytotoxic agents such as docetaxel may be more effective with fewer adverse events.
The prevalence of ILD and IPF in this study was 24.3 and 15.6 %, respectively, and seems to be higher than in previous reports (7.3–14 %) (Kinoshita et al. 2012; Nishino et al. 2015). However, among 387 patients who received surgical resection, 65 (16.8 %) were confirmed as underlying IPF (Goto et al. 2014). Location of the institution would greatly influence the prevalence of ILD and IPF as well as smoking status.
The limitations of this study are as follows. First, this was a retrospective, single-center study; a prospective, multicenter large study including both non-ILD and ILD patients is desirable to further investigate clinical differences in response to chemotherapy. Second, all patients were Japanese: Some studies have shown ethnic differences in incidence of drug-induced lung injury and other pulmonary diseases (Azuma et al. 2008; Natsuizaka et al. 2014). The incidence of AEs of ILD/IPF may vary between different ethnicities.
Conclusions
This study showed, for the first time, that patients with advanced NSCLC and IPF have shorter PFS and OS and lower DCR than patients without ILD. Docetaxel-containing chemotherapeutic regimens were more frequently associated with AEs than other regimens. Because survival after onset of AEs is usually short, more effective therapeutic strategies that do not induce AEs should be identified.
References
Azuma A, Hagiwara K, Kudoh S (2008) Basis of acute exacerbation of idiopathic pulmonary fibrosis in Japanese patients. Am J Respir Crit Care Med 177:1397–1398
Borchers AT, Chang C, Keen CL, Gershwin ME (2011) Idiopathic pulmonary fibrosis-an epidemiological and pathological review. Clin Rev Allergy Immunol 40:117–134
Chen YM, Shih JF, Perng RP, Tsai CM, Whang-Peng J (2006) A randomized trial of different docetaxel schedules in non-small cell lung cancer patients who failed previous platinum-based chemotherapy. Chest 129:1031–1038
Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, Lasky JA, Loyd JE, Noth I, Olman MA, Raghu G, Roman J, Ryu JH, Zisman DA, Hunninghake GW, Colby TV, Egan JJ, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kondoh Y, Lynch DA, Müller-Quernheim J, Myers JL, Nicholson AG, Selman M, Toews GB, Wells AU, Martinez FJ, Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators (2007) Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 176:636–643
Das S, Kumar M, Negi V, Pattnaik B, Prakash YS, Agrawal A, Ghosh B (2014) MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis. Am J Respir Cell Mol Biol 50:882–892
Fujimoto D, Tomii K, Otoshi T, Kawamura T, Tamai K, Takeshita J, Tanaka K, Matsumoto T, Monden K, Nagata K, Otsuka K, Nakagawa A, Hata A, Tachikawa R, Otsuka K, Hamakawa H, Katakami N, Takahashi Y, Imai Y (2013) Preexisting interstitial lung disease is inversely correlated to tumor epidermal growth factor receptor mutation in patients with lung adenocarcinoma. Lung Cancer 80:159–164
Goto T, Maeshima A, Oyamada Y, Kato R (2014) Idiopathic pulmonary fibrosis as a prognostic factor in non-small cell lung cancer. Int J Clin Oncol 19:266–273
Jiang X, Wang J, Zhang K, Tang S, Ren C, Chen Y (2015) The role of CD29-ILK-Akt signaling-mediated epithelial-mesenchymal transition of liver epithelial cells and chemoresistance and radioresistance in hepatocellular carcinoma cells. Med Oncol 32:141
Kanaji N, Basma H, Nelson A, Farid M, Sato T, Nakanishi M, Wang X, Michalski J, Li Y, Gunji Y, Feghali-Bostwick C, Liu X, Rennard SI (2014) Fibroblasts that resist cigarette smoke-induced senescence acquire profibrotic phenotypes. Am J Physiol Lung Cell Mol Physiol 307:L364–L373
Kanaji N, Okuda M, Dobashi H, Kameda T, Tadokoro A, Wakiya R, Kadowaki N, Bandoh S (2015) Squamous cell lung cancer associated with systemic sclerosis. J Clin Med Res 7:896–900
Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z (2005) TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res 6:56
Khalil N, Parekh TV, O’Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI (2001) Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax 56:907–915
Kinoshita T, Azuma K, Sasada T, Okamoto M, Hattori S, Imamura Y, Yamada K, Tajiri M, Yoshida T, Zaizen Y, Kawahara A, Fujimoto K, Hoshino T (2012) Chemotherapy for non-small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol Lett 4:477–482
Lee T, Park JY, Lee HY, Cho YJ, Yoon HI, Lee JH, Jheon S, Lee CT, Park JS (2014) Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med 108:1549–1555
Li J, Wang Y, Song Y, Fu Z, Yu W (2014) miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol Cancer 13:193
Li J, Liu H, Yu J, Yu H (2015) Chemoresistance to doxorubicin induces epithelial-mesenchymal transition via upregulation of transforming growth factor β signaling in HCT116 colon cancer cells. Mol Med Rep 12:192–198
Minegishi Y, Sudoh J, Kuribayasi H, Mizutani H, Seike M, Azuma A, Yoshimura A, Kudoh S, Gemma A (2011) The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer 71:70–74
Miyazono K (2009) Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci 85:314–323
Natsuizaka M, Chiba H, Kuronuma K, Otsuka M, Kudo K, Mori M, Bando M, Sugiyama Y, Takahashi H (2014) Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 190:773–779
Nishino M, Cardarella S, Dahlberg SE, Araki T, Lydon C, Jackman DM, Rabin MS, Hatabu H, Johnson BE (2015) Interstitial lung abnormalities in treatment-naïve advanced non-small-cell lung cancer patients are associated with shorter survival. Eur J Radiol 84:998–1004
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ, ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183:788–824
Raghu G, Lynch D, Godwin JD, Webb R, Colby TV, Leslie KO, Behr J, Brown KK, Egan JJ, Flaherty KR, Martinez FJ, Wells AU, Shao L, Zhou H, Pedersen PS, Sood R, Montgomery AB, O’Riordan TG (2014) Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: secondary analysis of a randomised, controlled trial. Lancet Respir Med 2:277–284
Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S, LUME-Lung 1 Study Group (2014) Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 15:143–155
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR, INPULSIS Trial Investigators (2014) Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370:2071–2082
Sakurada T, Kakiuchi S, Tajima S, Horinouchi Y, Okada N, Nishisako H, Nakamura T, Teraoka K, Kawazoe K, Yanagawa H, Nishioka Y, Minakuchi K, Ishizawa K (2015) Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother 49:398–404
Sato T, Watanabe A, Kondo H, Kanzaki M, Okubo K, Yokoi K, Matsumoto K, Marutsuka T, Shinohara H, Teramukai S, Kishi K, Ebina M, Sugiyama Y, Meinoshin O, Date H, Japanese Association for Chest Surgery (2015) Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 149:64–69
Selman M, Pardo A (2006) Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3:364–372
Shukuya T, Ishiwata T, Hara M, Muraki K, Shibayama R, Koyama R, Takahashi K (2010) Carboplatin plus weekly paclitaxel treatment in non-small cell lung cancer patients with interstitial lung disease. Anticancer Res 30:4357–4361
Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, Iglesias JL, Renschler MF (2012) Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 30:2055–2062
Sumikawa H, Johkoh T, Fujimoto K, Arakawa H, Colby TV, Fukuoka J, Taniguchi H, Kondoh Y, Kataoka K, Ogura T, Baba T, Ichikado K, Gyobu T, Yanagawa M, Honda O, Tomiyama N (2014) Pathologically proved nonspecific interstitial pneumonia: CT pattern analysis as compared with usual interstitial pneumonia CT pattern. Radiology 272:549–556
Tamiya A, Naito T, Miura S, Morii S, Tsuya A, Nakamura Y, Kaira K, Murakami H, Takahashi T, Yamamoto N, Endo M (2012) Interstitial lung disease associated with docetaxel in patients with advanced non-small cell lung cancer. Anticancer Res 32:1103–1106
Wang H, Sun Q, Wu Y, Wang L, Zhou C, Ma W, Zhang Y, Wang S, Zhang S (2015) Granzyme M expressed by tumor cells promotes chemoresistance and EMT in vitro and metastasis in vivo associated with STAT3 activation. Oncotarget 6:5818–5831
Watanabe N, Taniguchi H, Kondoh Y, Kimura T, Kataoka K, Nishiyama O, Kondo M, Hasegawa Y (2013) Efficacy of chemotherapy for advanced non-small cell lung cancer with idiopathic pulmonary fibrosis. Respiration 85:326–331
Watanabe N, Niho S, Kirita K, Umemura S, Matsumoto S, Yoh K, Ohmatsu H, Goto K (2015) Second-line docetaxel for patients with platinum-refractory advanced non-small cell lung cancer and interstitial pneumonia. Cancer Chemother Pharmacol 76:69–74
Wémeau-Stervinou L, Perez T, Murphy C, Polge AS, Wallaert B (2012) Lung capillary blood volume and membrane diffusion in idiopathic interstitial pneumonia. Respir Med 106:564–570
Acknowledgments
No funding was received specifically for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. No funding was received specifically for this study.
Human and animal rights
This study was approved by the Institutional Review Board of Kagawa University. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki revised at the 64th WMA General Assembly in 2013 or comparable ethical standards.
Informed consent
Informed consent was not obtained from all individual participants because this study is retrospective and includes patients who already died. However, our institute presented the study design officially which provided the chance all people check it and reject the participation in this study. This procedure was also approved by the Institutional Review Board of Kagawa University.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kanaji, N., Tadokoro, A., Kita, N. et al. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J Cancer Res Clin Oncol 142, 1855–1865 (2016). https://doi.org/10.1007/s00432-016-2199-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2199-z