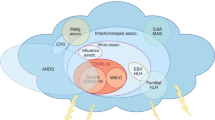
Abstract
Multisystemic inflammatory syndrome in children (MIS-C) is a rare, severe, post-infectious hyperinflammatory condition that occurs after COVID-19 infection. In this study, we aimed to demonstrate the risk reduction of MIS-C and severe MIS-C after Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccination. This nationwide cohort study included 526,685 PCR-confirmed COVID-19 cases (age < 19 years), of whom 14,118 were fully vaccinated prior to COVID-19 infection. MIS-C cases were collected from all hospitals in Israel from April 2020 through November 2021. The MIS-C rates were calculated among two COVID-19 populations: positive PCR confirmed cases and estimated COVID-19 cases (PCR confirmed and presumed). Vaccination status was determined from Ministry of Health (MoH) records. The MIS-C risk difference (RD) and 95% confidence intervals (95%CI) between vaccinated and unvaccinated patients are presented. Overall, 233 MIS-C cases under the age of 19 years were diagnosed and hospitalized in Israel during the study period. Among the estimated COVID-19 cases, MIS-C RD realistically ranged between 2.1 [95%CI 0.7–3.4] and 1.0 [95%CI 0.4–1.7] per 10,000 COVID-19 cases. For severe MIS-C, RD realistically ranged between 1.6 [95%CI 1.3–1.9] and 0.8 [95%CI 0.7–1.0], per 10,000 COVID-19 cases. Sensitivity analysis was performed on a wide range of presumed COVID-19 rates, demonstrating significant RD for each of these rates.
Conclusion: This research demonstrates that vaccinating children and adolescents against COVID-19 has reduced the risk of MIS-C during the study period.
What is Known: • Most of the published literature regarding vaccine effectiveness is based on case-control studies, which are limited due to small sample sizes and the inability to fully estimate the risk of MIS-C among vaccinated and unvaccinated children and adolescents. • The known underestimation of COVID-19 diagnosis among children and adolescents is challenging, as they often have few to no symptoms. | |
What is New: • Significant risk difference was found in favor of the vaccinated group, even after including extreme assumptions regarding the underdiagnosed COVID-19 rate. • During this nationwide study period, it was found that vaccinating children and adolescents reduced the risk of MIS-C and its complications. |


Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
Abbreviations
- CDC:
-
Centers for Disease Control
- CI:
-
Confidence intervals
- FDA:
-
Food and Drug Administration
- HMO:
-
Health Maintenance Organizations
- ICU:
-
Intensive care unit
- MoH:
-
Ministry of Health
- MIS-C:
-
Multisystemic inflammatory syndrome in children
- RD:
-
Risk difference
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- UK:
-
United Kingdom
- US:
-
United States
- VEU:
-
Vaccine emergency use
References
Feldstein LR, Tenforde MW, Friedman KG et al (2021) Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 325:1074–1087. https://doi.org/10.1001/jama.2021.2091
Feldstein LR, Rose EB, Horwitz SM et al (2020) Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 383:334–346. https://doi.org/10.1056/NEJMoa2021680
Riphagen S, Gomez X, Gonzalez-Martinez C et al (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395:1607–1608. https://doi.org/10.1016/S0140-6736(20)31094-1
HAN Archive - 00432 | Health Alert Network (HAN). https://emergency.cdc.gov/han/2020/han00432.asp. Accessed 26 Aug 2022
Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS -CoV-2 infection in children - France | ReliefWeb. https://reliefweb.int/report/france/rapid-risk-assessment-paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2?gclid=CjwKCAjw-IWkBhBTEiwA2exyO_JVOWgIOXeu5v5GqZ8jzCO5f-R327wqYV0A-1DW4esyFe1AZlWVnRoCRSMQAvD_BwE. Accessed 8 Jun 2023
Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) | CDC. https://www.cdc.gov/mis/mis-c/hcp/index.html. Accessed 26 Aug 2022
Nygaard U, Holm M, Hartling UB et al (2022) Incidence and clinical phenotype of multisystem inflammatory syndrome in children after infection with the SARS-CoV-2 delta variant by vaccination status: a Danish nationwide prospective cohort study. Lancet Child Adolesc Health 6:459–465. https://doi.org/10.1016/S2352-4642(22)00100-6
Yilmaz D, Ekemen Keles Y, Emiroglu M et al (2023) Evaluation of 601 children with multisystem inflammatory syndrome (Turk MISC study). Eur J Pediatr 182:5531–5542. https://doi.org/10.1007/s00431-023-05207-6
Lampidi S, Maritsi D, Charakida M et al (2024) Multisystem inflammatory syndrome in children (MIS-C): a nationwide collaborative study in the Greek population. Eur J Pediatr. https://doi.org/10.1007/s00431-023-05383-5
Payne AB, Gilani Z, Godfred-Cato S et al (2021) Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open 4:e2116420. https://doi.org/10.1001/jamanetworkopen.2021.16420
Karagiannidis C, Sander L-E, Mall MA, Busse R (2022) Incidence and outcomes of SARS-CoV-2-associated PIMS in Germany: a nationwide analysis. Infection. https://doi.org/10.1007/s15010-022-01877-w
Whittaker R, Greve-Isdahl M, Bøås H et al (2022) COVID-19 hospitalization among children <18 years by variant wave in Norway. Pediatrics. https://doi.org/10.1542/peds.2022-057564
Cohen JM, Carter MJ, Ronny Cheung C et al (2022) Lower risk of multisystem inflammatory syndrome in children (MIS-C) with the Delta and Omicron variants of SARS-CoV-2. Clin Infect Dis. https://doi.org/10.1093/cid/ciac553
FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine | FDA. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19. Accessed 8 Jun 2023
Coronavirus (COVID-19) update: FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in adolescents in another important action in fight against pandemic. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use. Accessed 16 Mar 2024
FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 years of age | FDA. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age. Accessed 26 Aug 2022
Lan Z, Yan J, Yang Y et al (2023) Effectiveness of COVID-19 vaccines among children and adolescents against SARS-CoV-2 variants: a meta-analysis. Eur J Pediatr 182:5235–5244. https://doi.org/10.1007/s00431-023-05216-5
Zambrano LD, Newhams MM, Olson SM et al (2022) Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep 71:52–58. https://doi.org/10.15585/mmwr.mm7102e1
Yu W, Guo Y, Zhang S et al (2022) Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 Omicron variant: a systematic review and analysis. J Med Virol 94:5790–5801. https://doi.org/10.1002/jmv.28066
Viner RM, Mytton OT, Bonell C et al (2021) Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr 175:143–156. https://doi.org/10.1001/jamapediatrics.2020.4573
Tsankov BK, Allaire JM, Irvine MA et al (2021) Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis 103:246–256. https://doi.org/10.1016/j.ijid.2020.11.163
Preston LE, Chevinsky JR, Kompaniyets L et al (2021) Characteristics and disease severity of US children and adolescents diagnosed with COVID-19. JAMA Netw Open 4:e215298. https://doi.org/10.1001/jamanetworkopen.2021.5298
Haas EJ, McLaughlin JM, Khan F et al (2022) Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis 22:357–366. https://doi.org/10.1016/S1473-3099(21)00566-1
Population - statistical abstract of Israel 2022 - No.73. https://www.cbs.gov.il/en/publications/Pages/2022/Population-Statistical-Abstract-of-Israel-2022-No.73.aspx. Accessed 8 Jun 2023
Zambrano LD, Newhams MM, Olson SM et al (2023) BNT162b2 mRNA vaccination against coronavirus disease 2019 is associated with a decreased likelihood of multisystem inflammatory syndrome in children aged 5–18 years-United States, July 2021 - April 2022. Clin Infect Dis 76:e90–e100. https://doi.org/10.1093/cid/ciac637
Reese H, Iuliano AD, Patel NN et al (2021) Estimated incidence of coronavirus disease 2019 (COVID-19) illness and hospitalization-United States, February-September 2020. Clin Infect Dis 72:e1010–e1017. https://doi.org/10.1093/cid/ciaa1780
Buonsenso D, Perramon A, Català M et al (2022) Multisystem inflammatory syndrome in children in Western countries? Decreasing incidence as the pandemic progresses?: an observational multicenter international cross-sectional study. Pediatr Infect Dis J 41:989–993. https://doi.org/10.1097/INF.0000000000003713
Munro APS, Jones CE, Faust SN (2024) Vaccination against COVID-19 - risks and benefits in children. Eur J Pediatr. https://doi.org/10.1007/s00431-023-05380-8
Laird-Gion J, Dionne A, Gauvreau K et al (2023) MIS-C across three SARS-CoV-2 variants: changes in COVID-19 testing and clinical characteristics in a cohort of U.S. children. Eur J Pediatr 182:2865–2872. https://doi.org/10.1007/s00431-023-04968-4
Acknowledgements
We thank Mr. Nati Brooks for initial support of the data management, Aviva Uliel for her assistance in data collection, Dr. Michal Stein and Dr. Tal Brosh for their assistance in determining the clinical status of borderline cases, and Karina Slobodkin for her role in data collection and building the database.
Author information
Authors and Affiliations
Contributions
S.A.P., N.S., R.R., S.R.S., and I.H. contributed to the design and implementation of the research. D.R.Z., J.W., and T.D. carried out the MIS-C case collection and confirmation. Analysis and manuscript write-up were carried out by N.S. and R.R., and all the authors aided in interpreting the results and worked on the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Israel Ministry of Health (protocol code 011-2022-MOH-COR). A waiver of informed consent form was granted as no identifiable patient data were mined and analyzed.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schwartz, N., Ratzon, R., Hazan, I. et al. Multisystemic inflammatory syndrome in children and the BNT162b2 vaccine: a nationwide cohort study. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05586-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05586-4