Abstract
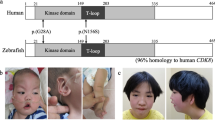
Cyclin-dependent kinase-like 5 (CDKL5) is a gene encoding a serine/threonine kinase that possesses an N-terminal catalytic domain and a large C-terminal domain and is located on the short arm of the X-chromosome at position 22 (Xp22). CDKL5 regulates neuronal migration, axonal growth, dendritic morphogenesis, and synaptic development and affects synaptic function. Pathogenic variants include deletions, truncations, splice variants, and missense variants. The specificity of CDKL5 is mainly determined by the shared sequence of amino acid residues, which is the phosphorylation site of the target protein with the motif Arg-Pro-X-Ser/Thr-Ala/Pro/Gly/Ser (R-P-X-[S/T]-[A/G/P/S]). Developmental encephalopathy caused by pathogenic variants of CDKL5 has a variety of nervous system symptoms, such as epilepsy, hypotonia, growth retardation, dyskinesia, cortical visual impairment, sleep disorders, and other clinical symptoms. This review summarizes the mechanism of CDKL5-induced allogeneic lesions in the nervous system and the clinical manifestations of related encephalopathy.
Conclusion: This review clarifies CDKL5's participation in neurodevelopmental diseases as well as its crucial function in dividing cells, cultured neurons, knockout mice, and human iPSC-derived neurons. CDKL5 variants help identify clinical diagnostic biomarkers. Although a few direct substrates of CDKL5 have been identified, more must be found in order to fully comprehend the signaling pathways connected to CDKL5 in the brain and the mechanisms that underlie its activities.
What is Known: • The CDKL5 gene has multiple functions and mainly causes disease by affecting neuronal migration, axon growth, dendritic morphogenesis, and synaptic development. Pathogenic variants include deletions, truncations, splice variants, and missense variants. Pathogenic missense mutations only occur in the kinase domain or have an impact on splicing sites. • Developmental encephalopathy caused by pathogenic variants of CDKL5 has a variety of nervous system symptoms, such as epilepsy, hypotonia, growth retardation, dyskinesia, cortical visual impairment, sleep disorders and other clinical symptoms. The CDKL5 deficiency disorder is being recognized as a severe developmental epileptic encephalopathy with early infancy start. | |
What is New: • Chemical genetic identification of CDKL5 substrates reveals its role in neuronal microtubule dynamics. Various MT-binding proteins have been identified as interactors of CDKL5. MTs are dynamic structures that are crucial for the migration, polarity, and shape of neurons. • Neurons in CDKL5 knockout neurons were kept alive by TGF-β1 restoration of SMAD3 signaling, providing evidence for a potential new therapeutic target for CDD. CDKL5 kinase activity is essential for the transcriptional silencing of genes induced by DNA double-strand breaks, CDKL5 may play a key role in the identification of DNA damage in neurons, and the discovery in understanding the molecular basis of CDKL5-related diseases is of great significance. |
Similar content being viewed by others
Abbreviations
- BAX:
-
Bcl2-associated X
- BDNF:
-
Brain-derived neurotrophic factor
- CDD:
-
CDKL5 deficiency
- CDKL5:
-
Cyclin-dependent kinase-like 5
- CEP131:
-
Centrosomal protein 131
- CLIP170:
-
Cytoplasmic linker protein 170
- ELOA:
-
Elongin A
- GEFs:
-
Guanine nucleotide exchange factors
- iPSCs:
-
Induced pluripotent stem cells
- MAP1S:
-
Microtubule-associated protein 1S
- MTs:
-
Microtubules
- NGL-1 :
-
Netrin G1 ligand
- NMDA:
-
N-methyl-D-aspartate
- PSD:
-
Postsynaptic density
- PSD-95:
-
Postsynaptic density-95
- Rac1:
-
Ras-related C3 botulinum toxin substrate 1
- RNAi:
-
RNA interference
- SMAD:
-
Small mothers against decapentaplegic
- SMAD3:
-
Small mothers against decapentaplegic 3
- TGF-β:
-
Transforming growth factor-β
- TIPS:
-
Terminal tracer proteins
- TrkB:
-
Tyrosine receptor kinase B
- Xp22:
-
X-chromosome at position 22
References
Montini E, Andolfi G, Caruso A, Buchner G, Walpole SM, Mariani M, Consalez G, Trump D, Ballabio A, Franco B (1998) Identification and characterization of a novel serine-threonine kinase gene from the Xp22 region. Genomics 51:427–433. https://doi.org/10.1006/geno.1998.5391
Negraes PD, Trujillo CA, Yu NK, Wu W, Yao H, Liang N, Lautz JD, Kwok E, McClatchy D, Diedrich J, de Bartolome SM, Truong J, Szeto R, Tran T, Herai RH, Smith SEP, Haddad GG, Yates JR 3rd, Muotri AR (2021) Altered network and rescue of human neurons derived from individuals with early-onset genetic epilepsy. Mol Psychiatry 26:7047–7068. https://doi.org/10.1038/s41380-021-01104-2
Barbiero I, Bianchi M, Kilstrup-Nielsen C (2022) Therapeutic potential of pregnenolone and pregnenolone methyl ether on depressive and CDKL5 deficiency disorders: focus on microtubule targeting. J Neuroendocrinol 34:e13033. https://doi.org/10.1111/jne.13033
Barbiero I, Peroni D, Siniscalchi P, Rusconi L, Tramarin M, De Rosa R, Motta P, Bianchi M, Kilstrup-Nielsen C (2020) Pregnenolone and pregnenolone-methyl-ether rescue neuronal defects caused by dysfunctional CLIP170 in a neuronal model of CDKL5 deficiency disorder. Neuropharmacology 164:107897. https://doi.org/10.1016/j.neuropharm.2019.107897
Baltussen LL, Negraes PD, Silvestre M, Claxton S, Moeskops M, Christodoulou E, Flynn HR, Snijders AP, Muotri AR, Ultanir SK (2018) Chemical genetic identification of CDKL5 substrates reveals its role in neuronal microtubule dynamics. The EMBO journal 37. https://doi.org/10.15252/embj.201899763
Erck C, Peris L, Andrieux A, Meissirel C, Gruber AD, Vernet M, Schweitzer A, Saoudi Y, Pointu H, Bosc C, Salin PA, Job D, Wehland J (2005) A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci USA 102:7853–7858. https://doi.org/10.1073/pnas.0409626102
Nirschl JJ, Magiera MM, Lazarus JE, Janke C, Holzbaur EL (2016) α-Tubulin tyrosination and CLIP-170 phosphorylation regulate the initiation of dynein-driven transport in neurons. Cell Rep 14:2637–2652. https://doi.org/10.1016/j.celrep.2016.02.046
Barbiero I, De Rosa R, Kilstrup-Nielsen C (2019) Microtubules: a key to understand and correct neuronal defects in CDKL5 deficiency disorder? Int J Mol Sci 20. https://doi.org/10.3390/ijms20174075
Barbiero I, Zamberletti E, Tramarin M, Gabaglio M, Peroni D, De Rosa R, Baldin S, Bianchi M, Rubino T, Kilstrup-Nielsen C (2022) Pregnenolone-methyl-ether enhances CLIP170 and microtubule functions improving spine maturation and hippocampal deficits related to CDKL5 deficiency. Hum Mol Genet 31:2738–2750. https://doi.org/10.1093/hmg/ddac067
Lansbergen G, Komarova Y, Modesti M, Wyman C, Hoogenraad CC, Goodson HV, Lemaitre RP, Drechsel DN, van Munster E, Gadella TW Jr, Grosveld F, Galjart N, Borisy GG, Akhmanova A (2004) Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J Cell Biol 166:1003–1014. https://doi.org/10.1083/jcb.200402082
Barbiero I, Peroni D, Tramarin M, Chandola C, Rusconi L, Landsberger N, Kilstrup-Nielsen C (2017) The neurosteroid pregnenolone reverts microtubule derangement induced by the loss of a functional CDKL5-IQGAP1 complex. Hum Mol Genet 26:3520–3530. https://doi.org/10.1093/hmg/ddx237
Urasaki A, Morishita S, Naka K, Uozumi M, Abe K, Huang L, Watase E, Nakagawa O, Kawakami K, Matsui T, Bessho Y, Inagaki N (2019) Shootins mediate collective cell migration and organogenesis of the zebrafish posterior lateral line system. Sci Rep 9:12156. https://doi.org/10.1038/s41598-019-48585-4
Nawaz MS, Giarda E, Bedogni F, La Montanara P, Ricciardi S, Ciceri D, Alberio T, Landsberger N, Rusconi L, Kilstrup-Nielsen C (2016) CDKL5 and Shootin1 interact and concur in regulating neuronal polarization. PloS one 11:e0148634. https://doi.org/10.1371/journal.pone.0148634
Ricciardi S, Ungaro F, Hambrock M, Rademacher N, Stefanelli G, Brambilla D, Sessa A, Magagnotti C, Bachi A, Giarda E, Verpelli C, Kilstrup-Nielsen C, Sala C, Kalscheuer VM, Broccoli V (2012) CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol 14:911–923. https://doi.org/10.1038/ncb2566
Luo L (2002) Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol 18:601–635. https://doi.org/10.1146/annurev.cellbio.18.031802.150501
Chen Q, Zhu YC, Yu J, Miao S, Zheng J, Xu L, Zhou Y, Li D, Zhang C, Tao J, Xiong ZQ (2010) CDKL5, a protein associated with rett syndrome, regulates neuronal morphogenesis via Rac1 signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:12777–12786. https://doi.org/10.1523/jneurosci.1102-10.2010
Zhu YC, Xiong ZQ (2019) Molecular and synaptic bases of CDKL5 disorder. Dev Neurobiol 79:8–19. https://doi.org/10.1002/dneu.22639
Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal RA (2007) Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron 55:53–68. https://doi.org/10.1016/j.neuron.2007.05.030
Muñoz IM, Morgan ME, Peltier J, Weiland F, Gregorczyk M, Brown FC, Macartney T, Toth R, Trost M, Rouse J (2018) Phosphoproteomic screening identifies physiological substrates of the CDKL5 kinase. The EMBO journal 37. https://doi.org/10.15252/embj.201899559
Baltussen LL, Rosianu F, Ultanir SK (2018) Kinases in synaptic development and neurological diseases. Prog Neuropsychopharmacol Biol Psychiatry 84:343–352. https://doi.org/10.1016/j.pnpbp.2017.12.006
Tegha-Dunghu J, Bausch E, Neumann B, Wuensche A, Walter T, Ellenberg J, Gruss OJ (2014) MAP1S controls microtubule stability throughout the cell cycle in human cells. J Cell Sci 127:5007–5013. https://doi.org/10.1242/jcs.136457
Halpain S, Dehmelt L (2006) The MAP1 family of microtubule-associated proteins. Genome Biol 7:224. https://doi.org/10.1186/gb-2006-7-6-224
Cao T, Matyas JJ, Renn CL, Faden AI, Dorsey SG, Wu J (2020) Function and mechanisms of truncated BDNF receptor TrkB.T1 in neuropathic pain. Cells 9. https://doi.org/10.3390/cells9051194
Sheng M, Hoogenraad CC (2007) The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem 76:823–847. https://doi.org/10.1146/annurev.biochem.76.060805.160029
Zhu YC, Li D, Wang L, Lu B, Zheng J, Zhao SL, Zeng R, Xiong ZQ (2013) Palmitoylation-dependent CDKL5-PSD-95 interaction regulates synaptic targeting of CDKL5 and dendritic spine development. Proc Natl Acad Sci USA 110:9118–9123. https://doi.org/10.1073/pnas.1300003110
Funke L, Dakoji S, Bredt DS (2005) Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem 74:219–245. https://doi.org/10.1146/annurev.biochem.74.082803.133339
Zhang Y, Matt L, Patriarchi T, Malik ZA, Chowdhury D, Park DK, Renieri A, Ames JB, Hell JW (2014) Capping of the N-terminus of PSD-95 by calmodulin triggers its postsynaptic release. EMBO J 33:1341–1353. https://doi.org/10.1002/embj.201488126
Choi Y, Park H, Jung H, Kweon H, Kim S, Lee SY, Han H, Cho Y, Kim S, Sim WS, Kim J, Bae Y, Kim E (2019) NGL-1/LRRC4C deletion moderately suppresses hippocampal excitatory synapse development and function in an input-independent manner. Front Mol Neurosci 12:119. https://doi.org/10.3389/fnmol.2019.00119
Taneja K, Ganesh S (2021) Dendritic spine abnormalities correlate with behavioral and cognitive deficits in mouse models of Lafora disease. J Comp Neurol 529:1099–1120. https://doi.org/10.1002/cne.25006
Tassinari M, Mottolese N, Galvani G, Ferrara D, Gennaccaro L, Loi M, Medici G, Candini G, Rimondini R, Ciani E, Trazzi S (2022) Luteolin treatment ameliorates brain development and behavioral performance in a mouse model of CDKL5 deficiency disorder. Int J Mol Sci 23. https://doi.org/10.3390/ijms23158719
Ren E, Roncacé V, Trazzi S, Fuchs C, Medici G, Gennaccaro L, Loi M, Galvani G, Ye K, Rimondini R, Aicardi G, Ciani E (2019) Functional and structural impairments in the perirhinal cortex of a mouse model of CDKL5 deficiency disorder are rescued by a TrkB agonist. Front Cell Neurosci 13:169. https://doi.org/10.3389/fncel.2019.00169
Schätzle P, Esteves da Silva M, Tas RP, Katrukha EA, Hu HY, Wierenga CJ, Kapitein LC, Hoogenraad CC (2018) Activity-dependent actin remodeling at the base of dendritic spines promotes microtubule entry. Current biology : CB 28:2081–2093.e6. https://doi.org/10.1016/j.cub.2018.05.004
Barbiero I, Valente D, Chandola C, Magi F, Bergo A, Monteonofrio L, Tramarin M, Fazzari M, Soddu S, Landsberger N, Rinaldo C, Kilstrup-Nielsen C (2017) CDKL5 localizes at the centrosome and midbody and is required for faithful cell division. Sci Rep 7:6228. https://doi.org/10.1038/s41598-017-05875-z
Hasenpusch-Theil K, Theil T (2021) The multifaceted roles of primary cilia in the development of the cerebral cortex. Frontiers in cell and developmental biology 9:630161. https://doi.org/10.3389/fcell.2021.630161
Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol 179:321–330. https://doi.org/10.1083/jcb.200707181
Di Nardo A, Rühmkorf A, Award P, Brennecke A, Fagiolini M, Sahin M (2022) Phenotypic characterization of Cdkl5-knockdown neurons establishes elongated cilia as a functional assay for CDKL5 deficiency disorder. Neurosci Res 176:73–78. https://doi.org/10.1016/j.neures.2021.10.001
Gennaccaro L, Fuchs C, Loi M, Pizzo R, Alvente S, Berteotti C, Lupori L, Sagona G, Galvani M, Gurgone A, Raspanti A, Medici G, Tassinari M, Trazzi S, Ren E, Rimondini R, Pizzorusso T, Zoccoli G, Giustetto M, Ciani E (2021) Age-related cognitive and motor decline in a mouse model of cDKL5 deficiency disorder is associated with increased neuronal senescence and death. Aging Dis 12:764–785. https://doi.org/10.14336/ad.2020.0827
Loi M, Trazzi S, Fuchs C, Galvani G, Medici G, Gennaccaro L, Tassinari M, Ciani E (2020) Increased DNA Damage and apoptosis in CDKL5-deficient neurons. Mol Neurobiol 57:2244–2262. https://doi.org/10.1007/s12035-020-01884-8
Khanam T, Muñoz I, Weiland F, Carroll T, Morgan M, Borsos BN, Pantazi V, Slean M, Novak M, Toth R, Appleton P, Pankotai T, Zhou H, Rouse J (2021) CDKL5 kinase controls transcription-coupled responses to DNA damage. The EMBO journal 40:e108271. https://doi.org/10.15252/embj.2021108271
Hiew LF, Poon CH, You HZ, Lim LW (2021) TGF-β/Smad signalling in neurogenesis: implications for neuropsychiatric diseases. Cells 10. https://doi.org/10.3390/cells10061382
Yu CY, Gui W, He HY, Wang XS, Zuo J, Huang L, Zhou N, Wang K, Wang Y (2014) Neuronal and astroglial TGFβ-Smad3 signaling pathways differentially regulate dendrite growth and synaptogenesis. NeuroMol Med 16:457–472. https://doi.org/10.1007/s12017-014-8293-y
Fuchs C, Medici G, Trazzi S, Gennaccaro L, Galvani G, Berteotti C, Ren E, Loi M, Ciani E (2019) CDKL5 deficiency predisposes neurons to cell death through the deregulation of SMAD3 signaling. Brain pathology (Zurich, Switzerland) 29:658–674. https://doi.org/10.1111/bpa.12716
Bahi-Buisson N, Kaminska A, Boddaert N, Rio M, Afenjar A, Gérard M, Giuliano F, Motte J, Héron D, Morel MA, Plouin P, Richelme C, V. des Portes, O. Dulac, C. Philippe, C. Chiron, R. Nabbout, T. Bienvenu, (2008) The three stages of epilepsy in patients with CDKL5 mutations. Epilepsia 49:1027–1037. https://doi.org/10.1111/j.1528-1167.2007.01520.x
Hong W, Haviland I, Pestana-Knight E, Weisenberg JL, Demarest S, Marsh ED, Olson HE (2022) CDKL5 deficiency disorder-related epilepsy: a review of current and emerging treatment. CNS Drugs 36:591–604. https://doi.org/10.1007/s40263-022-00921-5
Demarest ST, Olson HE, Moss A, Pestana-Knight E, Zhang X, Parikh S, Swanson LC, Riley KD, Bazin GA, Angione K, Niestroj LM, Lal D, Juarez-Colunga E, Benke TA (2019) CDKL5 deficiency disorder: relationship between genotype, epilepsy, cortical visual impairment, and development. Epilepsia 60:1733–1742. https://doi.org/10.1111/epi.16285
Olson HE, Demarest ST, Pestana-Knight EM, Swanson LC, Iqbal S, Lal D, Leonard H, Cross JH, Devinsky O, Benke TA (2019) Cyclin-dependent kinase-like 5 deficiency disorder: clinical review. Pediatr Neurol 97:18–25. https://doi.org/10.1016/j.pediatrneurol.2019.02.015
Fehr S, Leonard H, Ho G, Williams S, de Klerk N, Forbes D, Christodoulou J, Downs J (2015) There is variability in the attainment of developmental milestones in the CDKL5 disorder. J Neurodev Disord 7:2. https://doi.org/10.1186/1866-1955-7-2
Leonard H, Junaid M, Wong K, Demarest S, Downs J (2021) Exploring quality of life in individuals with a severe developmental and epileptic encephalopathy, CDKL5 Deficiency Disorder. Epilepsy research 169:106521. https://doi.org/10.1016/j.eplepsyres.2020.106521
Stosser MB, Lindy AS, Butler E, Retterer K, Piccirillo-Stosser CM, Richard G, McKnight DA (2018) High frequency of mosaic pathogenic variants in genes causing epilepsy-related neurodevelopmental disorders. Genetics in medicine : official journal of the American College of Medical Genetics 20:403–410. https://doi.org/10.1038/gim.2017.114
Fehr S, Wilson M, Downs J, Williams S, Murgia A, Sartori S, Vecchi M, Ho G, Polli R, Psoni S, Bao X, de Klerk N, Leonard H, Christodoulou J (2013) The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. European journal of human genetics : EJHG 21:266–273. https://doi.org/10.1038/ejhg.2012.156
Bahi-Buisson N, Bienvenu T (2012) CDKL5-related disorders: from clinical description to molecular genetics. Mol Syndromol 2:137–152. https://doi.org/10.1159/000331333
Fehr S, Wong K, Chin R, Williams S, de Klerk N, Forbes D, Krishnaraj R, Christodoulou J, Downs J, Leonard H (2016) Seizure variables and their relationship to genotype and functional abilities in the CDKL5 disorder. Neurology 87:2206–2213. https://doi.org/10.1212/wnl.0000000000003352
Fehr S, Downs J, Ho G, de Klerk N, Forbes D, Christodoulou J, Williams S, Leonard H (2016) Functional abilities in children and adults with the CDKL5 disorder. Am J Med Genet A 170:2860–2869. https://doi.org/10.1002/ajmg.a.37851
Bartnik M, Derwińska K, Gos M, Obersztyn E, Kołodziejska KE, Erez A, Szpecht-Potocka A, Fang P, Terczyńska I, Mierzewska H, Lohr NJ, Bellus GA, Reimschisel T, Bocian E, Mazurczak T, Cheung SW, Stankiewicz P (2011) Early-onset seizures due to mosaic exonic deletions of CDKL5 in a male and two females. Genetics in medicine : official journal of the American College of Medical Genetics 13:447–452. https://doi.org/10.1097/GIM.0b013e31820605f5
Frullanti E, Papa FT, Grillo E, Clarke A, Ben-Zeev B, Pineda M, Bahi-Buisson N, Bienvenu T, Armstrong J, Roche Martinez A, Mari F, Nissenkorn A, Lo Rizzo C, Veneselli E, Russo S, Vignoli A, Pini G, Djuric M, Bisgaard AM, Ravn K, Bosnjak VM, Hayek J, Khajuria R, Montomoli B, Cogliati F, Pintaudi M, Hadzsiev K, Craiu D, Voinova V, Djukic A, Villard L, Renieri A (2019) Analysis of the phenotypes in the Rett networked database. Int J Genomics 2019:6956934. https://doi.org/10.1155/2019/6956934
Jakimiec M, Paprocka J, Śmigiel R (2020) CDKL5 deficiency disorder-a complex epileptic encephalopathy. Brain sciences 10. https://doi.org/10.3390/brainsci10020107
Brock D, Fidell A, Thomas J, Juarez-Colunga E, Benke TA, Demarest S (2021) Cerebral visual impairment in CDKL5 deficiency disorder correlates with developmental achievement. J Child Neurol 36:974–980. https://doi.org/10.1177/08830738211019284
Quintiliani M, Ricci D, Petrianni M, Leone S, Orazi L, Amore F, Gambardella ML, Contaldo I, Veredice C, Perulli M, Musto E, Mercuri EM, Battaglia DI (2021) Cortical visual impairment in CDKL5 deficiency disorder. Front Neurol 12:805745. https://doi.org/10.3389/fneur.2021.805745
Wang Y, Peng W, Guo HY, Li H, Tian J, Shi YJ, Yang X, Yang Y, Zhang WQ, Liu X, Liu GN, Deng T, Sun YM, Xing WL, Cheng J, Feng ZC (2016) Next-generation sequencing-based molecular diagnosis of neonatal hypotonia in Chinese population. Sci Rep 6:29088. https://doi.org/10.1038/srep29088
Kobayashi Y, Tohyama J, Takahashi Y, Goto T, Haginoya K, Inoue T, Kubota M, Fujita H, Honda R, Ito M, Kishimoto K, Nakamura K, Sakai Y, Takanashi JI, Tanaka M, Tanda K, Tominaga K, Yoshioka S, Kato M, Nakashima M, Saitsu H, Matsumoto N (2021) Clinical manifestations and epilepsy treatment in Japanese patients with pathogenic CDKL5 variants. Brain Develop 43:505–514. https://doi.org/10.1016/j.braindev.2020.12.006
Mangatt M, Wong K, Anderson B, Epstein A, Hodgetts S, Leonard H, Downs J (2016) Prevalence and onset of comorbidities in the CDKL5 disorder differ from Rett syndrome. Orphanet J Rare Dis 11:39. https://doi.org/10.1186/s13023-016-0418-y
Heussler HS, Hiscock H (2018) Sleep in children with neurodevelopmental difficulties. J Paediatr Child Health 54:1142–1147. https://doi.org/10.1111/jpc.14164
Kadam SD, Sullivan BJ, Goyal A, Blue ME, Smith-Hicks C (2019) Rett syndrome and CDKL5 deficiency disorder: from bench to clinic. Int J Mol Sci 20. https://doi.org/10.3390/ijms20205098
Vigli D, Rusconi L, Valenti D, La Montanara P, Cosentino L, Lacivita E, Leopoldo M, Amendola E, Gross C, Landsberger N, Laviola G, Kilstrup-Nielsen C, Vacca RA, De Filippis B (2019) Rescue of prepulse inhibition deficit and brain mitochondrial dysfunction by pharmacological stimulation of the central serotonin receptor 7 in a mouse model of CDKL5 deficiency disorder. Neuropharmacology 144:104–114. https://doi.org/10.1016/j.neuropharm.2018.10.018
Eyers PA (2018) A new consensus for evaluating CDKL5/STK9-dependent signalling mechanisms. The EMBO journal 37. https://doi.org/10.15252/embj.2018100848
Funding
This work was supported by the Gansu Provincial Science and Technology Plan Project (21YF1FA171), the Lanzhou Science and Technology Plan Project (2018–1-111), the Second Hospital of Lanzhou University “Cuiying Technological Innovation” Program (CY2019-MS13), and the 2017 Lanzhou University Second Hospital “Cuiying Graduate Instructor” Training Program (201701).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Tiancheng Wang is responsible for the feasibility analysis, quality control, proofreading, supervision and management of the article. Xuyan Sun put forward the idea of the article, literature retrieval and collection, paper writing, and revision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Wang, T. Research progress on the pathogenesis of CDKL5 pathogenic variants and related encephalopathy. Eur J Pediatr 182, 3049–3056 (2023). https://doi.org/10.1007/s00431-023-05006-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05006-z