Abstract
The primary aim of this study was to document the treatment modalities used in periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome and look for the efficacy and safety of colchicine in the treatment of PFAPA patients. The secondary aim was to search for whether having MEFV (Mediterranean fever) gene sequence variants affect the clinical course and response to colchicine. The study was conducted in 2 pediatric rheumatology centers. The patients that have been diagnosed with PFAPA syndrome between December 2017 and December 2021 were evaluated retrospectively. The study included 157 patients with PFAPA syndrome (54.8% boys and 45.2% girls). The median follow-up duration was 18 (IQR: 12–30) months. One hundred and fifty-five patients (98.7%) had exudative pharyngitis, 120 patients (76.4%) had aphthous stomatitis, and 82 patients (52.2%) had cervical lymphadenitis during the attacks. Clinical features during attacks were not affected by the presence or absence of the MEFV gene sequence variants. Corticosteroid treatment during attacks was given to 152 patients (96.8%). The frequency of fever attacks did not change in 57 patients (37.5%), increased in 57 patients (37.5%), and decreased in 38 patients (25%) after corticosteroid use. Colchicine was given to 122 patients (77.7%) in the cohort. After colchicine treatment, complete/near-complete resolution of the attacks was observed in 57 patients (46.7%). Colchicine led to partial resolution of the attacks in 59 patients (48.4%). In only 6 patients (4.9%), no change was observed in the nature of the attacks with colchicine treatment. The median duration of the attacks was 4 (IQR: 4–5) days before colchicine treatment, and it was 2 (IQR: 1–2.5) days after colchicine treatment. Also, a significant decrease in the frequency of the attacks was observed before and after colchicine treatment [every 4 (IQR: 3–4) weeks versus every 10 (IQR: 8–24) weeks, respectively, (p < 0.001)]. The overall response to colchicine was not affected by MEFV sequence variants. It was seen that the frequency of fever attacks decreased dramatically in both groups, and children with MEFV variants had significantly less attacks than children without MEFV variants after colchicine treatment (every 11 weeks vs every 9.5 weeks, respectively, p: 0.02).
Conclusion: Colchicine seems to be an effective and safe treatment modality in PFAPA treatment. It led to a change in the nature of the attacks either in the frequency, duration, or severity of the attacks in 95.1% of the patients. This study has shown that having MEFV gene sequence variants did not affect the clinical course or response to colchicine. We recommend that colchicine should be considered in all PFAPA patients to see the response of the patient, irrespective of the MEFV gene mutations.
What is Known: • Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome is the most common periodic fever syndrome in the world. Familial Mediterranean fever (FMF) is the most common cause of periodic fever syndrome in Turkey. • Colchicine has become a new treatment option in PFAPA. | |
What is New: • Some PFAPA patients have Mediterranean fever (MEFV) gene variants, and it is speculated that PFAPA patients with MEFV gene mutations respond better to colchicine. • The aim of this study was to look for this hypothesis. We have seen that the clinical phenotype and colchicine response of PFAPA patients were not affected by MEFV gene sequence variants. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome was first described in 1987 by Marshall et al. [1]. The definition was modified in 1999 by Thomas et al. [2]. PFAPA is the most common disease in the group of periodic fever syndromes. It is diagnosed by the constellation of the clinical features. The disease is characterized by recurrent, stereotypic attacks of fever, aphthous stomatitis, exudative tonsillopharyngitis, and painful cervical lymphadenitis. The attacks tend to recur every 3–6 weeks, and each attack lasts for 3–6 days [1, 2]. It is not a monogenic disease, and yet, the etiopathogenesis of the disease has not been fully elucidated [3]. The diagnosis necessitates the exclusion of other causes of recurrent fevers in children (i.e., cyclic neutropenia, immune deficiencies, infections, and other monogenic periodic fever syndromes) [1, 2]. The disease does not cause any long-term morbidity like amyloidosis, and it tends to resolve spontaneously towards the end of the 1st decade. But it affects the quality of life of the patients and their families tremendously. There is no standardized treatment modality due to the lack of randomized controlled trials. The use of corticosteroids during attacks and tonsillectomy are the main treatment modalities [4,5,6].
The use of a single dose of corticosteroid (prednisolone 1–2 mg/kg/dose, single dose) during an attack and resolution of the fever in a couple of hours is an important clue for diagnosis [5]. In some patients, administration of corticosteroids may decrease the interval between attacks and may increase in others [4, 5]. Tonsillectomy seems to be the most curative treatment modality in PFAPA [1, 5]. It has 80–85% success rate, but some families are hesitant to tonsillectomy due to fear of complications [7]. Prophylactic use of cimetidine and colchicine may decrease the number of attacks [8,9,10,11,12,13,14,15,16].
The primary aim of this study was to document the treatment modalities used in PFAPA and look for the efficacy and safety of colchicine in the treatment of PFAPA patients. The secondary aim was to search for whether having MEFV (Mediterranean fever) gene sequence variants affect the clinical course and response to colchicine.
Materials and methods
The study was conducted in 2 pediatric rheumatology centers between December 2017 and December 2021. All patients in the cohort fulfilled the modified Marshall criteria and Eurofever/Paediatric Rheumatology International Trials Organisation (PRINTO) classification criteria for autoinflammatory recurrent fevers [2, 4]. The patients with less than 6 months of follow-up were excluded from the study.
Demographics (gender, age at the first symptom, age at diagnosis, follow-up duration, and family history), frequency and duration of the attacks, clinical features during attacks (oral aphthous, tonsillopharyngitis, cervical lymphadenitis), MEFV gene results, treatment modalities used (corticosteroids, colchicine, tonsillectomy), and outcomes of the treatments were recorded from the files.
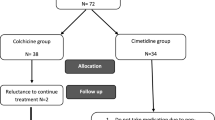
All treatment modalities were discussed with the families at the time of diagnosis and each control visit. Mutual decisions were made for each patient. Corticosteroid use during attacks was the first treatment modality used in the cohort. Colchicine treatment was used in children that have increased or the same numbers of attacks after corticosteroid use. Colchicine was used for at least 6 months before calling the patient as colchicine unresponsive. The colchicine dose used was 0.5 mg/day for children ≤ 5 years of age, 1 mg/day for children 5–10 years of age, and 1.5 mg/day for children > 10 years of age. A modified version of the CARRA PFAPA Consensus Treatment Plan Workgroup response criteria was used to define colchicine response [5]. Complete response was defined as the total resolution of the attacks. Near complete response was defined as ≥ 80% decrease in the number of attacks. Partial response was defined as either < 80% decrease in the number of the attacks or any decrease in the days or degree of fever. Cimetidine is not found in our country, so it was not used in any patient. Tonsillectomy was performed in patients that had unchanged frequency of attacks after corticosteroids and/or colchicine use.
The study was approved by the local ethics committee and was performed according to the tenets of the Declaration of Helsinki. Written informed consent was received from the legal guardians of the children.
The SPSS version 21.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The variables were investigated using visual (histogram, probability plots) and analytic methods (Kolmogorov–Smirnov/Shapiro–Wilk’s test) to determine whether or not they were normally distributed. Quantitative data with non-normal distribution were presented as the median and interquartile range (IQR). Categorical data were presented as counts and percentages. Categorical variables were compared with the Chi-square test or Fisher’s exact test where appropriate. The Mann–Whitney U test was used to compare the non-normally distributed continuous data between two groups. Non-parametric Wilcoxon test was used for quantitative data (frequency and duration of attacks) before and after colchicine treatment. A p-value < 0.05 was considered significant.
Results
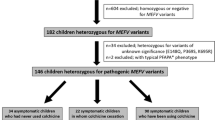
The files of 173 PFAPA patients were reviewed for the study. Thirteen patients due to the short observation period and 3 patients because of the missing data were excluded from the study. The study included 157 patients with PFAPA (86 males, 71 females). The median age at the first symptom was 18 (IQR: 9–30) months, and the median age at the time of diagnosis was 36 (IQR: 24–48) months. The median duration of follow-up was 18 (IQR: 12–30) months. The median delay in diagnosis was 12 (IQR: 8–22) months.
In the cohort, 155 patients (98.7%) had pharyngitis, 120 patients (76.4%) had aphthous ulcers, and 82 patients (52.2%) had cervical lymphadenitis during the attacks. The median frequency of the attacks was 4 (IQR: 3–4) weeks, and the median duration of fevers was 4 (IQR: 4–5) days.
The MEFV gene analysis was performed to all patients in the cohort (exons 2, 3, 5, and 10). In 46 (29.3%) children, heterozygous sequence variants were found in the MEFV gene. None of the patients in the study had homozygous or compound heterozygous pathogenic mutations in the MEFV gene and did not have clinical features of FMF. The clinical features of patients with and without MEFV gene variants and MEFV gene results are given in Table 1. The delay in diagnosis and months of follow-up were higher in patients with MEFV mutation than in those without (p < 0.001). However, clinical features during the attacks, frequency, and duration of the attacks were similar in both groups.
The outcome of corticosteroid treatment
A single dose of prednisolone (1–2 mg/kg/dose) during attacks was used in 152 patients (96.8%). The parents of the remaining 5 patients did not want to use corticosteroids during attacks. Corticosteroid use led to the resolution of fever in that attack in all patients. The frequency of fever attacks did not change in 57 patients (37.5%), increased in 57 patients (37.5%), and decreased in 38 patients (25%).
The outcome of colchicine treatment
Colchicine was used in 122 patients (77.7%). The median duration of colchicine use was 15 (IQR: 10–22) months. After colchicine treatment, complete/near-complete resolution of the attacks was observed in 57 patients (46.7%). Colchicine led to partial resolution of the attacks in 59 patients (48.4%). In only 6 patients (4.9%), no change was observed in the nature of the attacks with colchicine treatment. In the whole cohort, the median duration of the attacks was 4 (IQR: 4–5) days before colchicine treatment, and it was 2 (IQR: 1–2.5) days after colchicine treatment. Also, a significant decrease in the frequency of the attacks was observed before and after colchicine treatment [every 4 (IQR: 3–4) weeks versus every 10 (IQR: 8–24) weeks, respectively, (p < 0.001)].The colchicine response segregated by MEFV gene sequence variants is given in Table 2. It was seen that the frequency of fever attacks decreased dramatically in both groups, and children with MEFV variants had significantly less attacks than children without MEFV variants after colchicine treatment (every 11 weeks vs every 9.5 weeks, respectively, p = 0.02). A decrease in the attack duration was found to be similar in both groups. The overall response to colchicine was not affected by MEFV sequence variants. Colchicine-related side effect was observed only in 4 patients (3.2%). Three patients had mild and transient abdominal pain and diarrhea, and one patient had transient leucopenia (white blood cell count: 3300/mm3). Adverse events disappeared with dose reduction and did not recur after normalization of the dose. During the study period, no colchicine-related severe adverse events were observed.
The outcome of tonsillectomy
Tonsillectomy was performed in 12 (7.6%) patients. These 12 children in the tonsillectomy arm were older and had longer duration of follow-up than 145 patients who did not have tonsillectomy (the mean current age: 7.6 versus 4.5 years, p < 0.001; the mean follow-up duration: 35.5 months versus 18 months, p < 0.001, respectively). Tonsillectomy resulted in complete resolution of the attacks in 4 (33.3%) patients and partial resolution of the attacks in 3 (25%) patients. In 5 (41.7%) patients, tonsillectomy did not modify the disease course at all. In 2 patients, colchicine was used after the failure of tonsillectomy and led to complete resolution of the attacks.
Discussion
This study evaluated the efficacy of colchicine in children with PFAPA. It was seen that colchicine was effective in 95.1% of the patients. It led to a significant amount of decrease in the number of attacks and also the duration of fever days during the attacks. We have seen that 29.2% of the PFAPA patients had heterozygous MEFV gene sequence variants, but neither the clinical features nor the response to the colchicine was affected by having MEFV gene variants. Children with MEFV gene variants had a more pronounced decrease in the frequency of the attacks.
PFAPA is the most common cause of recurrent fever in children. Due to the lack of randomized controlled trials, there is no standard, universally accepted treatment modality for PFAPA. There are mostly expert opinion-based recommendations for the management of PFAPA [5]. The use of corticosteroids during an attack is very effective in the termination of that attack, but it does not prevent the recurrence of the attacks [17]. It is also known that corticosteroids may decrease (i.e., from every month to every 2–3 months) or even increase (i.e., from every month to every 2–3 weeks) the number of the attacks [18]. In this study, the use of corticosteroids decreased the frequency of the attacks in 25% and increased the frequency of the attacks in 37.5% of the patients.
Familial Mediterranean fever is the most common monogenic periodic fever syndrome. Since FMF and PFAPA have recurrent fever as the common manifestation, diagnosis of PFAPA may be problematic in countries where FMF is endemic [12, 19,20,21,22]. Recent studies have shown that patients with PFAPA have increased interleukin-1 production during attacks, like FMF patients [23, 24]. Heterozygous MEFV variants have been reported in patients with PFAPA. Furthermore, it was reported that having MEFV variants may alter the disease course and results of the treatment modalities [8,9,10,11,12, 25, 26]. The frequency of having MEFV variants in our cohort was 29.2%. In the study of Güneş et al. [11], 96% of patients with PFAPA with MEFV variants responded well to colchicine, while this ratio was 80% in patients without MEFV variants (p = 0.003). They pointed out that children with MEFV gene variants had a better response to the colchicine prophylaxis. Yildiz et al. [27] found MEFV gene variants in 59.9% of PFAPA patients, and they postulated that these variants may change the onset of disease and duration of the attacks. But they have observed that having MEFV variants did not affect the clinical course or colchicine response in their study. The carrier status for FMF is around 20% in our country [28]. The high rate of having MEFV variants in PFAPA patients in our study and also in other studies from our country could be explained by this high rate of carrier status in the healthy population. The study of Batu et al. [29] has shown that the clinical picture of PFAPA patients may change with the geographical location, independent of MEFV gene variants. They reported that the differences between patients from the Turkish and US cohorts may be due to epigenetic or environmental factors, but the phenotype of PFAPA patients was similar between patients with and without MEFV variants. In our study, we have also observed that having MEFV gene sequence variants did not affect the clinical phenotype of PFAPA patients.
The efficacy of colchicine in PFAPA has been reported in the previous studies (Table 3) [8,9,10,11,12,13,14]. In the study of Tasher et al. [9], colchicine was used in 9 patients, and the frequency of the attacks decreased from every 1.7 weeks to every 8.4 weeks (p < 0.006). In a multicenter French study, 9 of 20 PFAPA patients (45%) responded well to colchicine, and 4/9 patients (71%) had heterozygous MEFV variants [10]. The study of Butbul Aviel et al. [8] was a randomized, placebo-controlled trial of colchicine in PFAPA. The study included 18 children (10 patients in the control group and 8 patients in the study group) with 6 months of follow-up. In the 6th month, children using colchicine had less attacks than the control group (4.9 vs 1.6, p ≤ 0.001). Six of the 8 patients (75%) using colchicine had MEFV variants. In a retrospective study from Turkey, Güneş et al. [11] used colchicine in 356 of 400 PFAPA patients for 1 year. They stated that colchicine was effective in 85% of the patients by decreasing the frequency of the attacks from every 18.8 days to every 49.5 days. In the same study, it was observed that 96% of patients with MEFV variants and 80% of patients without MEFV mutations responded to the colchicine treatment.
Pehlivan et al. [12] reported the results of a similar study, and PFAPA patients having MEFV variants responded better to colchicine than patients without MEFV variants (66% vs 33%, respectively, p = 0.003). In the study of Wetzel et al. [13], 21 of 27 PFAPA patients (77.7%) responded well to colchicine. Quintana-Ortega et al. [14] gave 12 months results of colchicine treatment in 13 PFAPA patients. The median number of flares in 12 months decreased from 8 to 3 (p = 0.005), and the median duration of the attacks decreased from 4 days to 1 day (p = 0.003). Also, the highest degree of fever during attacks decreased from 40 to 38.5 °C (p = 0.002). They stated that colchicine could be an effective treatment option in PFAPA patients with frequent or severe attacks. Similar results with colchicine have been observed in our study. In 95.1% of the patients, we have seen some degree of clinical response; either a decrease in the frequency of the attacks, duration of the attacks, or degree of the fever. In nearly half of the patients, we have seen a dramatic decrease in the number of attacks. Contrary to the literature, we have seen that response to the colchicine was not affected by the presence of MEFV gene sequence variants.
Tonsillectomy has been the main treatment modality in PFAPA for many years. The cure rate is around 80–90% [30, 31]. In recent years, tonsillectomy has been less commonly performed in PFAPA. Vigo et al. [32] reported the long-term, a mean of 54.5 months, follow-up results of 275 PFAPA patients. Tonsillectomy was performed in 41 (14.9%) patients. Remission rates were found to be similar in patients with tonsillectomy and medical therapy (65.9% vs 59.1%, p = 0.51). Yıldız et al. [33] reported a higher ratio of tonsillectomy and remission rates after tonsillectomy. Tonsillectomy was performed in 313 (51.5%) of 607 patients, and attacks ceased in 95.4% of the patients after the surgery. Tonsillectomy was chosen as a treatment modality only in 7.6% of the patients in our cohort, and complete resolution of the attacks was observed in 33.3% of the patients after tonsillectomy. The good clinical response of the patients to the colchicine treatment was the main reason for the low number of patients with tonsillectomy. The second reason for not choosing tonsillectomy was the pandemic of COVID-19. All elective surgeries were canceled for a long time in our country, as in the whole world.
The main weakness of the study was its retrospective design. We have excluded patients with missing data or short follow-up time to create a more homogenous cohort. The main strength of the study was that it included a large number of PFAPA patients with MEFV gene analysis results.
In conclusion, the results of this study have shown that colchicine is an effective and safe treatment modality in children with PFAPA. It should be considered in PFAPA patients that have frequent attacks before deciding on tonsillectomy. We recommend that it should be used for at least 6 months before calling the child colchicine unresponsive. We have also seen that the clinical phenotype and response to colchicine were not affected by having MEFV gene sequence variants, so MEFV gene results in PFAPA patients should be interpreted cautiously.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Abbreviations
- FMF:
-
Familial Mediterranean fever
- IQR:
-
Interquartile range
- MEFV:
-
Mediterranean fever
- PFAPA:
-
Periodic fever, aphthous stomatitis, pharyngitis, and adenitis
References
Marshall GS, Edwards KM, Butler J, Lawton AR (1987) Syndrome of periodic fever, pharyngitis, and aphthous stomatitis. J Pediatr 110:43–46. https://doi.org/10.1016/s0022-3476(87)80285-8
Thomas KT, Feder HM Jr, Lawton AR, Edwards KM (1999) Periodic fever syndrome in children. J Pediatr 135:15–21. https://doi.org/10.1016/s0022-3476(99)70321-5
Asna Ashari K, Rezaei N (2021) PFAPA (periodic fever, aphthous stomatitis, pharyngitis, and adenitis) syndrome: an overview of genetic background. Clin Rheumatol 40:4437–4444. https://doi.org/10.1007/s10067-021-05770-z
Gattorno M, Hofer M, Federici S, Vanoni F, Bovis F, Aksentijevich I, Anton J, Arostegui JI, Barron K, Ben-Cherit E, Brogan PA, Cantarini L, Ceccherini I, De Benedetti F, Dedeoglu F, Demirkaya E, Frenkel J, Goldbach-Mansky R, Gul A, Hentgen V, Hoffman H, Kallinich T, Kone-Paut I, Kuemmerle-Deschner J, Lachmann HJ, Laxer RM, Livneh A, Obici L, Ozen S, Rowczenio D, Russo R, Shinar Y, Simon A, Toplak N, Touitou I, Uziel Y, van Gijn M, Foell D, Garassino C, Kastner D, Martini A, Sormani MP, Ruperto N; Eurofever Registry and the Paediatric Rheumatology International Trials Organisation (PRINTO) (2019) Classification criteria for autoinflammatory recurrent fevers Ann Rheum Dis 78:1025-1032. https://doi.org/10.1136/annrheumdis-2019-215048
Amarilyo G, Rothman D, Manthiram K, Edwards KM, Li SC, Marshall GS, Yildirim-Toruner C, Haines K, Ferguson PJ, Lionetti G, Cherian J, Zhao Y, DeLaMora P, Syverson G, Nativ S, Twilt M, Michelow IC, Stepanovskiy Y, Thatayatikom A, Harel L, Akoghlanian S, Tucker L, Marques MC, Srinivasalu H, Propst EJ, Licameli GR, Dedeoglu F, Lapidus S, CARRA PFAPA Consensus Treatment Plan Workgroup (2020) Consensus treatment plans for periodic fever, aphthous stomatitis, pharyngitis and adenitis syndrome (PFAPA): a framework to evaluate treatment responses from the childhood arthritis and rheumatology research alliance (CARRA) PFAPA work group. Pediatr Rheumatol Online J 18:31. https://doi.org/10.1186/s12969-020-00424-x
Gaggiano C, Rigante D, Sota J, Grosso S, Cantarini L (2019) Treatment options for periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome in children and adults: a narrative review. Clin Rheumatol 38:11–17. https://doi.org/10.1007/s10067-018-4361-2
Batu ED, Batu HB (2019) Recurrence of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome after tonsillectomy: case-based review. Rheumatol Int 39:1099–1105. https://doi.org/10.1007/s00296-019-04310-y
Butbul Aviel Y, Tatour S, Gershoni Baruch R, Brik R (2016) Colchicine as a therapeutic option in periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome. Semin Arthritis Rheum 45:471–474. https://doi.org/10.1016/j.semarthrit.2015.07.005
Tasher D, Stein M, Dalal I, Somekh E (2008) Colchicine prophylaxis for frequent periodic fever, aphthous stomatitis, pharyngitis and adenitis episodes. Acta Paediatr 97:1090–1092. https://doi.org/10.1111/j.1651-2227.2008.00837.x
Dusser P, Hentgen V, Neven B, Koné-Paut I (2016) Is colchicine an effective treatment in periodic fever, aphtous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome? Joint Bone Spine 83:406–411. https://doi.org/10.1016/j.jbspin.2015.08.017
Gunes M, Cekic S, Kilic SS (2017) Is colchicine more effective to prevent periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis episodes in Mediterranean fever gene variants? Pediatr Int 59:655–660. https://doi.org/10.1111/ped.13265
Pehlivan E, Adrovic A, Sahin S, Barut K, Kul Cınar O, Kasapcopur O (2018) PFAPA Syndrome in a population with endemic familial Mediterranean fever. J Pediatr 192:253–255. https://doi.org/10.1016/j.jpeds.2017.08.078
Welzel T, Ellinghaus M, Wildermuth AL, Deschner N, Benseler SM, Kuemmerle-Deschner JB (2021) Colchicine effectiveness and safety in periodic fever, aphthous stomatitis, pharyngitis, and adenitis. Front Pediatr 9:759664. https://doi.org/10.3389/fped.2021.759664
Quintana-Ortega C, Seoane-Reula E, Fernández L, Camacho M, Olbrich P, Neth O, Murias S, Udaondo C, Remesal A, Calvo C, Alcobendas R (2020) Colchicine treatment in children with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: a multicenter study in Spain. Eur J Rheumatol 8:73–78. https://doi.org/10.5152/eurjrheum.2020.20102
Hofer M (2020) Why and how should we treat periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome? Paediatr Drugs 22:243–250. https://doi.org/10.1007/s40272-020-00393-4
Sparud-Lundin C, Berg S, Fasth A, Karlsson A, Wekell P (2019) From uncertainty to gradually managing and awaiting recovery of a periodic condition- a qualitative study of parents’ experiences of PFAPA syndrome. BMC Pediatr 19:99. https://doi.org/10.1186/s12887-019-1458-y
Tasher D, Somekh E, Dalal I (2006) PFAPA syndrome: new clinical aspects disclosed. Arch Dis Child 91:981–984. https://doi.org/10.1136/adc.2005.084731
Vanoni F, Theodoropoulou K, Hofer M (2016) PFAPA syndrome: a review on treatment and outcome. Pediatr Rheumatol Online J 14:38. https://doi.org/10.1186/s12969-016-0101-9
Öztürk K, Coşkuner T, Baglan E, Sönmez HE, Yener GO, Çakmak F, Demirkan FG, Tanatar A, Karadag SG, Ozdel S, Demir F, Çakan M, Aktay Ayaz N, Sözeri B (2022) Real-life data from the largest pediatric familial Mediterranean fever cohort. Front Pediatr 9:805919. https://doi.org/10.3389/fped.2021.805919
Özen S, Batu ED, Demir S (2017) Familial Mediterranean fever: recent developments in pathogenesis and new recommendations for management. Front Immunol 8:253. https://doi.org/10.3389/fimmu.2017.00253
Adrovic A, Sahin S, Barut K, Kasapcopur O (2019) Familial Mediterranean fever and periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome: shared features and main differences. Rheumatol Int 39:29–36. https://doi.org/10.1007/s00296-018-4105-2
Butbul Aviel Y, Harel L, Abu Rumi M, Brik R, Hezkelo N, Ohana O, Amarilyo G (2019) Familial Mediterranean fever is commonly diagnosed in children in Israel with periodic fever aphthous stomatitis, pharyngitis, and adenitis syndrome. J Pediatr 204:270–274. https://doi.org/10.1016/j.jpeds.2018.08.080
Kolly L, Busso N, von Scheven-Gete A, Bagnoud N, Moix I, Holzinger D, Simon G, Ives A, Guarda G, So A, Morris MA, Hofer M (2013) Periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome is linked to dysregulated monocyte IL-1β production. J Allergy Clin Immunol 131:1635–1643. https://doi.org/10.1016/j.jaci.2012.07.043
Stojanov S, Hoffmann F, Kéry A, Renner ED, Hartl D, Lohse P, Huss K, Fraunberger P, Malley JD, Zellerer S, Albert MH, Belohradsky BH (2006) Cytokine profile in PFAPA syndrome suggests continuous inflammation and reduced anti-inflammatory response. Eur Cytokine Netw 17:90–97
Dagan E, Gershoni-Baruch R, Khatib I, Mori A, Brik R (2010) MEFV, TNF1rA, CARD15 and NLRP3 mutation analysis in PFAPA. Rheumatol Int 30:633–636. https://doi.org/10.1007/s00296-009-1037-x
Taniuchi S, Nishikomori R, Iharada A, Tuji S, Heike T, Kaneko K (2013) MEFV Variants in patients with PFAPA syndrome in Japan. Open Rheumatol J 7:22–25. https://doi.org/10.2174/1874312901307010022
Yildiz M, Adrovic A, Ulkersoy I, Gucuyener N, Koker O, Sahin S, Haslak F, Barut K, Kasapcopur O (2021) The role of Mediterranean fever gene variants in patients with periodic fever, aphthous stomatitis, pharyngitis, and adenitis syndrome. Eur J Pediatr 180:1051–1058. https://doi.org/10.1007/s00431-020-03840-z
Yilmaz E, Ozen S, Balci B, Duzova A, Topaloglu R, Besbas N, Saatci U, Bakkaloglu A, Ozguc M (2001) Mutation frequency of familial Mediterranean fever and evidence for a high carrier rate in the Turkish population. Eur J Hum Genet 9:553–555. https://doi.org/10.1038/sj.ejhg.5200674
Batu ED, Kara Eroğlu F, Tsoukas P, Hausmann JS, Bilginer Y, Kenna MA, Licameli GR, Fuhlbrigge RC, Özen S, Dedeoğlu F (2016) Periodic fever, aphthosis, pharyngitis, and adenitis syndrome: analysis of patients from two geographic areas. Arthritis Care Res (Hoboken) 68:1859–1865. https://doi.org/10.1002/acr.22901
Garavello W, Romagnoli M, Gaini RM (2009) Effectiveness of adenotonsillectomy in PFAPA syndrome: a randomized study. J Pediatr 155:250–253. https://doi.org/10.1016/j.jpeds.2009.02.038
Burton MJ, Pollard AJ, Ramsden JD, Chong LY, Venekamp RP (2014) Tonsillectomy for periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis syndrome (PFAPA). Cochrane Database Syst Rev 9:CD008669. https://doi.org/10.1002/14651858.CD008669.pub2
Vigo G, Martini G, Zoppi S, Vittadello F, Zulian F (2014) Tonsillectomy efficacy in children with PFAPA syndrome is comparable to the standard medical treatment: a long-term observational study. Clin Exp Rheumatol 32(4 Suppl 84):S156–S159
Yıldız M, Haslak F, Adrovic A, Ülkersoy İ, Gücüyener N, Şahin S, Barut K, Kasapçopur Ö (2022) Periodic fever, aphthous stomatitis, pharyngitis, and adenitis syndrome: a single-center experience. Turk Arch Pediatr 57:46–52. https://doi.org/10.5152/TurkArchPediatr.2021.21229
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by GOY, İA, CAM, and MÇ. The first draft of the manuscript was written by GOY and MÇ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the local ethics committee (date: 29 September 2021, number: HRU/21.21.01) and was performed according to the tenets of the Declaration of Helsinki. Written informed consent was received from the legal guardians of the children.
Consent to participate
Informed consent was obtained from the legal guardians of the children.
Consent for publication
Informed consent for publication was obtained from the legal guardians of the children.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Tobias Tenenbaum
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Otar Yener, G., Aktaş, İ., Altıntaş Meşe, C. et al. Does having MEFV gene sequence variants affect the clinical course and colchicine response in children with PFAPA syndrome?. Eur J Pediatr 182, 411–417 (2023). https://doi.org/10.1007/s00431-022-04709-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04709-z