Abstract
The threshold to initiate empiric antibiotics for suspicion of early-onset sepsis (EOS) is low in preterm infants. Antibiotics’ effects on short-term outcomes have recently been debated. We aimed at exploring the extent of early empiric antibiotic exposure (EEAE) in preterm infants and the association between the duration of EEAE with necrotizing enterocolitis (NEC) and late-onset sepsis (LOS) within different EEAE groups. EEAE practice for suspicion of EOS was evaluated in all included infants (gestational age < 30 weeks) born in 9 centers in the Netherlands and Belgium between Oct. 2014 and Jan. 2019. EEAE association with NEC and LOS development was analyzed by multivariate regression. After excluding 56 EOS cases, 1259 infants were included. A total of 1122 infants (89.1%) were exposed to empirical antibiotics for the suspicion of EOS of whom 802 (63.7%) had short (≤ 72 h) and 320 (25.4%) prolonged EEAE (> 72 h). Infants with EEAE ≤ 72 h had a lower incidence of NEC compared to both infants without EEAE (adjusted odds ratio (aOR) 0.39; 95% confidence interval (CI) [0.19–0.80]; p = 0.01) and with prolonged EEAE (> 72 h) (aOR [95%CI]: 0.58 [0.35–0.96]; p = 0.03). With every additional day of EEAE, LOS incidence decreased (aOR [95%CI]: 0.90 [0.85–0.97]; p = 0.003).
Conclusion: Almost 90% of preterm infants who have negative blood culture results in the first 72 h of life are exposed to EEAE under suspicion of EOS. One-fourth has prolonged EEAE. Duration of EEAE was differently associated with NEC and LOS incidence. The effects of antibiotics, and potentially induced microbial dysbiosis related to development of NEC and LOS, should further be explored.
What is Known: • Preterm infants often receive antibiotics empirically directly after birth for suspicion of early-onset sepsis. • The effects of the duration of early empirical antibiotic exposure on the risk for necrotizing enterocolitis and late-onset sepsis are debated. | |
What is New: • Almost 90% of preterm infants with a gestational age below 30 weeks are exposed to antibiotics empirically after birth despite negative culture results. In a quarter of these culture-negative infants, empirical antibiotics are prolonged. • A short course of empirical antibiotics (≤72h) is associated with decreased odds for necrotizing enterocolitis compared to both prolonged (>72h) or no empirical antibiotics after birth. Furthermore, every additional day of empirical antibiotic exposure is associated with decreased risk for late-onset sepsis in the first month of life. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal sepsis remains one of the leading causes of morbidity and mortality at the neonatal intensive care unit (NICU) [1]. Given the high burden associated with delayed treatment of early-onset sepsis (EOS), threshold for empiric initiation of antibiotics is low in preterm infants [2]. Consequently, over 75% of very low birth weight (VLBW; birth weight < 1500 g) infants are empirically exposed to antibiotics [3]. Empirical therapy is usually discontinued upon negative blood culture results after 48–72 h. However, as blood culture has low sensitivity, the course is often prolonged out of fear of undertreating clinical sepsis [2, 4].
Potential adverse effects of antibiotic exposure include antibiotic resistance and dysregulation of microbial gut colonization by decreasing the diversity and promoting overgrowth of potential pathogens [5]. Specifically at neonatal age, early empiric antibiotic exposure (EEAE) has been suggested to increase the risk of long-term adverse effects, such as development of metabolic and auto-immune disorders [5]. In the short term, it has been demonstrated in VLBW infants that every additional day of antibiotic exposure is associated with worse composite outcomes of multiple adverse events, including necrotizing enterocolitis (NEC) and late-onset sepsis (LOS) [6]. However, these findings have recently been questioned by observational and animal model studies, suggesting a mitigating effect of antibiotics on NEC [7, 8]. In murine models, antibiotics decrease bloodstream infections, potentially by delaying colonization, lowering the bacterial load at the level of the intestinal mucosa and the load of invasive microorganisms at the epithelial border [9].
This hypothesis is supported by two recent case–control studies performed by our group, showing that antibiotic exposure was associated with decreased odds of gram-positive LOS and, when initiated directly postpartum, with decreased odds of NEC [10, 11]. Neither study, however, focused specifically on EEAE for EOS suspicion and both were prone to confounding by indication, as antibiotic treatment and extension thereof could depend on clinical factors, which are also associated with NEC and LOS.
In the current larger multicenter cohort study, we aim to explore clinical characteristics associated with (prolongation of) EEAE and investigate the association between the duration of EEAE with NEC and LOS.
Materials and methods
Study design and participants
This study was embedded in an ongoing prospective multicenter preterm cohort study in nine participating NICUs in the NetherlandsFootnote 1 and Belgium,Footnote 2 with the primary objective of identifying novel non-invasive biomarkers, as well as clinical risk factors, for LOS and NEC in the first 28 days of life [12]. Consequently, included participants have, in part, been described in previous case–control studies investigating fecal biomarkers and a wide range of risk factors for LOS and NEC [10, 11]. In our current study, we included all infants born before 30 weeks of gestation between October 2014 and July 2019 whose parents provided informed consent (Ethical Board permission A2020.190). Antibiotics for risk or suspicion of EOS were started by the attending physician in standard dosage and administered parenterally, according to the NICE guideline on Antibiotics for early-onset neonatal infection [13]. None of the participating centers routinely prescribed probiotics in the study period.
We excluded infants with major congenital malformations, including gastrointestinal malformation, such as anal or intestinal atresia and Hirschsprung’s disease [10, 11]. Additionally, in accordance with previous research, we excluded infants with culture-proven EOS and infants demised in the first week of life, irrespective of the cause of death [6, 14, 15]. Infants with culture-proven EOS were excluded since they require prolonged treatment with antibiotics, thus not being treated empirically. Finally, inaccessibility to patient record data on antibiotic exposure and morbidity was an additional exclusion criterion.
Definitions
EEAE was defined as antibiotic exposure started within the first 72 h of life under the suspicion of EOS, but in the absence of a positive blood and, if applicable, cerebrospinal fluid culture. The duration was counted per started 24 h. Common antibiotic practice per center for suspicion of EOS with included number of participants is presented in Table S1. Subjects were categorized based on EEAE duration: (1) no EEAE; (2) short EEAE (≤ 72 h); or (3) prolonged EEAE (> 72 h). The cut-off point of 72 h was chosen in agreement with common clinical practice, where empiric antibiotic therapy is often discontinued within 48–72 h in case clinical and biochemical correlates for sepsis are missing [16].
Infants were classified as NEC cases, when diagnosed with NEC stage IIA or higher, according to the modified Bell’s staging criteria [17]. All infants with NEC were independently reviewed by two experts (TM, HN) for classification. In case of discrepancy, infants were reevaluated until an agreement was reached. All neonatal LOS episodes, defined as blood culture–proven sepsis with onset beyond the first 72 h and within the first 28 days, were analyzed and classified (Supplementary Table S2) [18, 19]. Infants could be classified as both NEC and LOS cases if they met the criteria for both.
Feeding practice was subcategorized as done previously, consisting of three categories: (1) human milk (HM), either own mother’s milk (MM) or donor milk (DM), (2) formula feeding (FM), (3) combination of HM and FM (Table S2) [11]. The highest C-reactive protein level within 72 h after birth was recorded. Inotropic medication and type of ventilation support were registered between 48 and 72 h after birth, as the decision whether to prolong empirical antibiotics is made at that moment. Standard demographic and clinical data were collected. Additional definitions of clinical and demographic characteristics are depicted in Table S2.
Statistical analysis
Statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) version 26.0 (IBM, Armonk, NY, USA). Continuous demographic and clinical characteristics were depicted, depending on normality, as either mean and standard deviation (SD) or median and interquartile range [IQR] for the three groups of interest: (1) no EEAE, (2) short (≤ 72 h), and (3) prolonged (> 72 h) EEAE. Where appropriate, continuous data were analyzed by parametric one-way ANOVA, or non-parametric Kruskal–Wallis tests. The normal distribution of continuous data was assessed visually. Categorical data were analyzed by Pearson’s chi-squared test. Two-sided p-values of < 0.05 were considered statistically significant.
Associations between EEAE and incidence of NEC and LOS were analyzed by univariate and multivariate logistic regression methods with EEAE as a dichotomous variable (unexposed versus exposed infants). Secondly, the duration of EEAE was analyzed both as a categorical variable (no EEAE vs. short (≤ 72 h) vs. prolonged (> 72 h EEAE), and as a continuous variable (EEAE in number of calendar days).
In the multivariate models, odds ratios (ORs) were adjusted for confounding variables previously associated with NEC and LOS development [11, 20]: center of birth, gestational age, birth weight percentiles, gender, mode of delivery, invasive ventilation and/or inotropic medication use at day two of life, and type of enteral feeding. For LOS, a 5-min Apgar score and duration of parenteral feeding were added. Results from the logistic regression were reported as OR and adjusted OR (aOR), along with the respective 95% confidence interval (95%CI). Subgroup analyses for coagulase-negative staphylococcus (CoNS) and non-CoNS sepsis were additionally performed.
A post hoc uni- and multivariate analysis was performed to assess odds for LOS and non-CoNS LOS after exclusion of all LOS cases who were diagnosed before the postnatal age of 7 days. Although the most common definition of LOS is sepsis with onset ≥ 72 h of life, some clinicians, as well as several studies, define LOS as sepsis beyond the first week of life [19, 21]. With this analysis, we aimed at ensuring the comparability of our methods with those studies.
Results
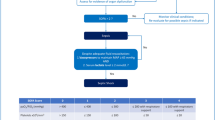
A total of 1490 infants born before 30 weeks of gestation were screened for eligibility between October 2014 and January 2019, of whom 231 were excluded. The main reasons for exclusion were lack of informed consent (n = 159) and culture-proven EOS (n = 56). Additional motives for exclusion are depicted in Fig. 1.
Of the 1259 included infants with negative blood culture results from the first 72 h of life, 1122 (89%) had EEAE for the suspicion of EOS, of whom 802 (64%) had short EEAE (≤ 72 h) and 320 (25%) prolonged EEAE (Fig. 1). Prolonged EAEE ranged between 19 and 44%, depending on the center of birth (Table S1).
Baseline characteristics are depicted in Table 1. Infants without EEAE were more often born by caesarean section and were smaller for gestational age (SGA), while infants with prolonged EEAE were invasively ventilated, needed inotropic medication, and had an increased CRP level (≥ 10 mg/dl) more often than the other groups.
In the first 28 days of life, NEC occurred in 107 infants (8.4%), of whom 40 needed surgical intervention. LOS was diagnosed in 421 (33.4%) neonates, of which 192 were caused by a non-CoNS pathogen. The median age of onset of NEC was comparable between EEAE groups, while LOS occurred at a later age with increasing EEAE duration (Fig. 2A and B, resp., Table 1). Incidence of NEC and LOS by EEAE duration are represented graphically in Supplementary Fig. S1A–C and D–E, resp.
When corrected for confounding factors, the odds of NEC were lower in infants with any EEAE, compared to no EEAE (aOR 0.47; 95%CI 0.23–0.96; p = 0.04). Short (≤ 72 h) EEAE was associated with lower odds of developing NEC, compared to both no EEAE (aOR 0.39; 95%CI 0.19–0.80; p = 0.01) and prolonged (> 72 h) EEAE (aOR 0.58; 95%CI 0.35–0.96; p = 0.03) (Table 3). EEAE duration as a continuous variable could not be analyzed in relation to NEC incidence as the linearity assumption for logistic regression analysis was not met, regardless of data transformation or non-linear term addition.
LOS was diagnosed in 421 of the 1259 infants (33.4%). The median onset of LOS differed significantly between EEAE groups (Table 1). Table 2 demonstrates the incidences of LOS subtypes, based on causative pathogens and type of LOS. No differences were found in overall LOS incidence between infants with and without EEAE (Table 3). However, EEAE was associated with a lower incidence of non-CoNS LOS, compared to non-exposure to antibiotics (aOR 0.49; 95%CI 0.25–0.96; p = 0.04) (Table S3). Only prolonged EEAE, but not short EEAE, was associated with lower non-CoNS LOS incidence, compared to no EEAE (aOR 0.35; 95%CI 0.16–0.74; p = 0.007) (Supplementary Table S3).
When antibiotic exposure was analyzed as a continuous variable (number of days of exposure), a lower LOS incidence was found for every additional day of EEAE (aOR 0.90; 95%CI 0.85–0.97; p = 0.003). This negative association with the duration of empirical antibiotic exposure was observed in all subcategories of LOS (Table 3; Supplementary Table S3).
Post hoc analysis was performed solely on sepsis cases diagnosed beyond the first week. As analyzed by univariate logistic regression, prolonged EEAE was associated with higher odds for LOS, compared to both short and no EEAE. When corrected for confounding factors, this association could not be observed (Supplementary Table S4).
Discussion/conclusion
The continuation of early empiric antibiotics despite negative blood culture results, and its effect on short-term outcomes, is debated [5, 7,8,9]. In this prospective multicenter cohort study, we observed that the vast majority of preterm infants are empirically exposed to antibiotics directly after birth. In about one-quarter of infants, antibiotics were continued empirically beyond 72 h, despite negative cultures. Infants with prolonged EEAE were of lower gestational age and were more often intubated, receiving inotropic medication and had higher CRP values in the first 72 h of life. They, however, had lower adjusted odds of developing LOS, compared to infants without EEAE. The group without EEAE, moreover, had higher adjusted odds of developing NEC, relative to the short EEAE group, but similar adjusted odds of NEC compared to infants with prolonged EEAE.
Similar to our findings, several studies have reported an increased risk for NEC with prolonged EEAE, compared to short EEAE [22,23,24]. On the contrary, the recent NEOMUNE study including 2831 VLBW infants did not demonstrate a significant difference in NEC incidence in the short antibiotic exposure (≤ 72 h) group versus the prolonged exposure (> 72 h) group: 4.3% vs. 3.7% [7]. However, they did report a lower NEC incidence (3.9%) following any early antibiotic exposure in comparison to non-exposed infants (9%) (OR 0.25, 95% 0.12–0.47, p < 0.001). Notably, the study population consisted of over 90% of infants receiving antibiotic treatment, of whom the majority received prolonged antibiotic treatment (> 72 h), as opposed to our cohort, in which a short course was more common. Moreover, there was a disproportionally large amount of infants born small for gestational age (SGA) and/or by caesarean section in the group of infants without EEAE, both of which are known risk factors for NEC [25]. Even though the outcome was statistically corrected for this potential confounding by indication, residual confounding may still be present. This limitation could not be avoided in our current study.
Other studies including sufficiently large groups of preterm infants not exposed to antibiotics are scarce, but our findings are further corroborated by experimental studies on preterm piglets, showing that no EEAE was associated with a higher incidence of NEC compared to EEAE [8]. EEAE resulted in increased mucosal integrity and decreased inflammatory responses, suggesting potential protective mechanisms of early antibiotics exposure on the preterm gut through immune modulation related to early gut microbiota colonization [8]. It is hypothesized that this protective mechanism could result from a delay in intestinal colonization with potential pathogens [7]. Because of this delayed colonization of pathogenic bacteria, the intestinal immune defense system might be stimulated towards postnatal adaptation [8, 9]. However, this potential beneficial effect might be negated by prolonged EEAE, as this might provoke NEC by perturbed microbial colonization [26].
One small RCT including 22 preterm infants supports the hypothesis of a protective role of short EEAE, as a more favorable microbial composition was found in infants who were randomized to 48 h of antibiotic treatment versus no EEAE [27]. Kim et al. [27] reported an increased abundance of Actinobacteriota (formerly Actinobacteria), which was largely contributed by Bifidobacteriaceae, in the EEAE group, a family previously associated with a decreased risk of NEC [28]. Notably, increased Actinobacteriota have also been associated with NEC in other studies, however in combination with significantly decreased abundance of Bifidobacteriaceae. The REASON trial, a small RCT comparing a short course of antibiotics to no antibiotics, did not show a difference in microbiota between the treatment and control arm and concluded that the difference in microbiota was largely attributable to feeding type [29].
The potential protective role of EEAE for LOS that is suggested by our results, and those of el Manouni el Hassani et al. [11], is not supported by current literature on humans. Kuppala et al. [21], e.g., reported a positive association between every additional day of antibiotic exposure and LOS incidence in a preterm cohort. Their study design, however, differed in terms of follow-up period—120 days, compared to 28 days in the current study—and in terms of the study population—infants developing LOS in the first week of life were excluded by the research group [21]. In the current study, a post hoc analysis was performed excluding sepsis cases with onset < 7 days. In line with Kuppala et al.’s [21] results, unadjusted odds for LOS were lower for the non-EEAE group, compared to the prolonged EEAE group. After adjustments for confounding factors, there was no association between the duration of EEAE and LOS incidence. In our opinion, the exclusion of infants developing LOS in the first week of life might be subjected to bias, especially given that more than half of the infants who developed LOS in our non-EEAE group were diagnosed within the first week of life (median age of LOS onset 6 days). As the median age of LOS onset in the short and prolonged EEAE group was 9 and 11 days, respectively, exclusion of all LOS cases would proportionately exclude more infants with LOS in the non-EEAE group, thus underestimating LOS onset in this group. This could, however, not entirely explain the difference in results as two larger studies including 587 and 4039 infants, respectively, which did include early LOS cases during the first week of life also found a higher LOS incidence with increasing antibiotics administration [14, 30].
Although both NEC and (non-CoNS) LOS are preceded by intestinal dysbiosis [31, 32], the contrast between NEC and LOS incidence in association with EEAE might suggest different pathophysiology regarding gut microbiota-related immune responses. Despite the fact that antibiotic administration could stimulate immune maturation [33], this might not be equally relevant for different diseases and should further be explored.
The current observational study has several strengths, including the multicenter design, the large cohort size, and the prospective collection of detailed data on daily basis, allowing adjustment for relevant clinical and demographic factors. This also allowed us to study NEC and LOS separately and not as a combined outcome as was previously done in some studies [14, 34]. The categorization of participants based on antibiotic duration allowed us to identify non-linear associations between the duration of antibiotic exposure and NEC.
This study has several limitations, next to those characteristic of observational studies. Despite that several differences in baseline characteristics were corrected for in the multivariate analysis, there remains a risk of residual confounding of unidentified factors. Furthermore, obstetrical data could not be accessed, missing data on pre-eclampsia, umbilical cord blood flow, and intrapartum antibiotic treatment, potentially leading to underestimation of the infants’ antibiotic exposure. Registration was discontinued after the 28th day of life, which could have led to missing some LOS cases. As the first LOS episode usually occurs within the first weeks of life, we hypothesized that the number of missed cases would be limited [35].
Further research on EEAE and health effects is warranted. Future perspectives include larger RCTs aiming at unravelling the effects of EEAE in low-risk infants for EOS. For example, results from the NICU Antibiotics and Outcomes (NANO) trial (ClinicalTrials.gov identifier: NCT03997266) are needed to identify the suggested (protective) effect of empirical antibiotics for NEC and LOS and to identify the optimal duration of empirical antibiotics. The interaction of antibiotics with other factors influencing the early gut colonization and immunity should be investigated. It remains to be elucidated whether current strategies against NEC, e.g., enteral feeding with human milk and the use of probiotics, have a synergistic preventive effect when combined with (short) EEAE or whether EEAE might rather be more helpful in a subgroup receiving formula feeding [36]. Studies should additionally take a broad spectrum of potential short- and long-term adverse events into account [37, 38]. In parallel, microbiota studies, preferably by metagenomics analysis, should be performed in infants receiving different lengths of empirical antibiotics to assess short- and long-term effects on intestinal colonization. In the future, these insights could allow for targeted microbiota-based preventive strategies in an optimally selected population and time window for improving the development of the immature gut [9].
Despite our findings, we believe that providing more antibiotics than currently advised, e.g., a standard short-term administration of empiric antibiotics (48–72 h) instead of watchful waiting without antibiotics in case of low risk of early-onset sepsis, should not be advised. First, the plethora of potential antibiotic-related adverse events, such as increased antibiotic resistance and other short- and long-term effects, should be further investigated [5, 39]. Current guidelines on antibiotic stewardship should be followed until results on RCTs assessing the effects of EEAE, such as the abovementioned NANO trial, are published. Empirical antibiotics should only be started when there is substantial suspicion or high risk of EOS and discontinued as soon as deemed safe (in absence of positive blood culture and reassuring clinical picture).
In conclusion, in this multicenter cohort, almost 90% of preterm infants with negative postnatal blood cultures was exposed to empirical antibiotics for suspicion of EOS. Twenty-five percent had prolonged (> 72 h) empirical exposure. A short (≤ 72 h) empirical course of antibiotics was associated with a decreased risk for NEC compared to no antibiotics and a prolonged antibiotic course. On the other hand, prolonged EEAE was associated with a decreased risk for LOS in the first 28 days of life, compared to no antibiotics. Potential antibiotic-induced changes in microbiome composition and function and their association with NEC and LOS development should be explored in future studies.
Data availability
The data used in the current study are not publicly available but are available upon reasonable request.
Notes
Netherlands: Amalia Children’s Hospital, Radboud; Amalia Children’s Center, Zwolle; Beatrix Children’s Hospital, Groningen; Emma Children’s Hospital (location AMC & location Vumc), Amsterdam; Maastricht University Medical Center, Maastricht; Máxima Medical Center, Veldhoven; Wilhelmina Children’s Hospital, Utrecht.
Belgium: University Hospitals Leuven, Leuven.
Abbreviations
- AB:
-
Antibiotics
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- CoNS:
-
Coagulase-negative staphylococcus
- CRP:
-
C-reactive protein
- DM:
-
Donor milk
- EEAE:
-
Early empiric antibiotic exposure: antibiotics initiated in the first 72 h of life for the suspicion of early-onset sepsis
- EOS:
-
Early-onset sepsis
- FM:
-
Formula milk
- HM:
-
Human milk (mother’s own milk or donor milk)
- LOS:
-
Late-onset sepsis
- MM:
-
Mother’s milk
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatal intensive care unit
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- SGA:
-
Small for gestational age
- SPSS:
-
Statistical Package for Social Sciences
- VLBW:
-
Very low birthweight
References
Stoll BJ et al (2011) Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 127(5):817–26
Klingenberg C et al (2018) Culture-negative early-onset neonatal sepsis - at the crossroad between efficient sepsis care and antimicrobial stewardship. Front Pediatr 6:285–285
Mukhopadhyay S, Sengupta S, Puopolo KM (2019) Challenges and opportunities for antibiotic stewardship among preterm infants. Archives of disease in childhood. Fetal Neonatal Ed 104(3):F327-F332
Mundal HS et al (2021) Antibiotic use in term and near-term newborns. Pediatrics 148(6)
Becattini S, Taur Y, Pamer EG (2016) Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med 22(6):458–478
Ting JY et al (2019) Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics 143(3)
Li Y et al (2020) Early use of antibiotics is associated with a lower incidence of necrotizing enterocolitis in preterm, very low birth weight infants: the NEOMUNE-NeoNutriNet Cohort Study. J Pediatr
Jiang P et al (2012) Antibiotics increase gut metabolism and antioxidant proteins and decrease acute phase response and necrotizing enterocolitis in preterm neonates. PLoS ONE 7(9):e44929
Nguyen DN et al (2016) Oral antibiotics increase blood neutrophil maturation and reduce bacteremia and necrotizing enterocolitis in the immediate postnatal period of preterm pigs. Innate Immun 22(1):51–62
Berkhout DJC et al (2018) Risk factors for necrotizing enterocolitis: a prospective multicenter case-control study. Neonatology 114(3):277–284
El Manouni El Hassani S et al (2019) Risk factors for late-onset sepsis in preterm infants: a multicenter case-control study. Neonatology 116(1):42–51
Berkhout DJC et al (2018) The potential of gut microbiota and fecal volatile organic compounds analysis as early diagnostic biomarker for necrotizing enterocolitis and sepsis in preterm infants. Expert Rev Gastroenterol Hepatol 12(5):457–470
NICEguidelines (2013) Neonatal infection: antibiotics for prevention and treatment. National Institute for Health and Care Excellence (NG195)
Cotten CM et al (2009) Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 123(1):58–66
Greenberg RG et al (2019) Prolonged duration of early antibiotic therapy in extremely premature infants. Pediatr Res 85(7):994–1000
Meem M et al (2011) Biomarkers for diagnosis of neonatal infections: a systematic analysis of their potential as a point-of-care diagnostics. J Glob Health 1(2):201–209
Kliegman RM, Walsh MC (1987) Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 17(4):213–288
Investigators of the Vermont-Oxford Trials Network Database Project (1993) The Vermont-Oxford Trials Network: very low birth weight outcomes for 1990. Pediatrics 91(3):540–5
Dong Y, Speer CP (2015) Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed 100(3):F257–F263
Samuels N et al (2017) Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr 17(1):105–105
Kuppala VS et al (2011) Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr 159(5):720–725
Esaiassen E et al (2017) Antibiotic exposure in neonates and early adverse outcomes: a systematic review and meta-analysis. J Antimicrob Chemother 72(7):1858–1870
Esmaeilizand R et al (2018) Antibiotic exposure and development of necrotizing enterocolitis in very preterm neonates. Paediatr Child Health 23(4):e56–e61
Alexander VN, Northrup V, Bizzarro MJ (2011) Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr 159(3):392–397
Ree IM et al (2014) Necrotizing enterocolitis in small-for-gestational-age neonates: a matched case-control study. Neonatology 105(1):74–78
Fjalstad JW et al (2018) Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: a systematic review. J Antimicrob Chemother 73(3):569–580
Kim CS et al (2021) Effect of antibiotic use within first 48 hours of life on the preterm infant microbiome: a randomized clinical trial. JAMA Pediatr 175(3):303–305
Hagen PC, Skelley JW (2019) Efficacy of bifidobacterium species in prevention of necrotizing enterocolitis in very-low birth weight infants. A systematic review. J Pediatr Pharmacol Ther JPPT: Official J PPAG 24(1):10–15
Russell JT et al (2021) Antibiotics and the developing intestinal microbiome, metabolome and inflammatory environment in a randomized trial of preterm infants. Sci Rep 11(1):1943
Alsafadi T et al (2018) Does prolonged initial empirical antibiotics treatment increase morbidity and mortality in preterm infants & #60;34 weeks? J Clin Neonatol 7(3):116–120
el Manouni el Hassani S et al (2021) Profound pathogen-specific alterations in intestinal microbiota composition precede late onset sepsis in preterm infants: a longitudinal multicenter case-control study. Clin Infect Dis
Masi AC et al (2020) Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut
Nguyen DN et al (2016) Delayed development of systemic immunity in preterm pigs as a model for preterm infants. Sci Rep 6:36816–36816
Cantey JB et al (2018) Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J Pediatr 203:62–67
Letouzey M et al (2022) Early antibiotic exposure and adverse outcomes in very preterm infants at low risk of early-onset sepsis: the EPIPAGE-2 Cohort Study. J Pediatr 243:91–98 e4
Siggers RH et al (2011) Nutritional modulation of the gut microbiota and immune system in preterm neonates susceptible to necrotizing enterocolitis. J Nutr Biochem 22(6):511–521
Cantey JB (2020) Early antibiotic therapy and adverse outcomes in preterm infants: time for a trial! J Pediatr 227:13–14
Varkas G et al (2018) Association of inflammatory bowel disease and acute anterior uveitis, but not psoriasis, with disease duration in patients with axial spondyloarthritis: results from two Belgian nationwide axial spondyloarthritis cohorts. Arthritis Rheumatol 70(10):1588–1596
Cotten CM (2016) Adverse consequences of neonatal antibiotic exposure. Curr Opin Pediatr 28(2):141–149
Funding
This work was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement [grant number 814168] and from The Stichting Zeldzame Ziekten Fonds [grant number N/A]. The funding sources had no role in the design and conduct of the study.
Author information
Authors and Affiliations
Contributions
Thomas Dierikx, Nancy Deianova, and Jip Groen designed the study, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. Daniel Vijlbrief, Veerle Cossey, Christian Hulzebos, Esther d’Haens, Boris Kramer, Willem de Boode, Mirjam van Weissenbruch, Wouter de Jonge, Marc Benninga, Chris van den Akker, Anton van Kaam, and Douwe Visser conceptualized and designed the collection, collected data, and critically reviewed the manuscript for important intellectual content. Nanne de Boer and Hendrik Niemarkt conceptualized and designed the study and data collection, collected data, and critically reviewed the manuscript for important intellectual content. Tim de Meij conceptualized and designed the study and data collection, collected data, supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the local institutional review board of Amsterdam UMC, location VUmc, Amsterdam, the Netherlands (approval number A2020.190).
Consent to participate
Written informed consent was obtained from both parents and/or legal guardians of all infants.
Competing interests
N. de Boer has served as a speaker for AbbVie, Takeda, and MSD. He has served as a consultant and principal investigator for Takeda and/or TEVA Pharma B.V. He has received research grants from Dr. Falk, MLDS, and Takeda. All outside the submitted work. All other authors declare not to have relevant financial or non-financial interests to disclose.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dierikx, T.H., Deianova, N., Groen, J. et al. Association between duration of early empiric antibiotics and necrotizing enterocolitis and late-onset sepsis in preterm infants: a multicenter cohort study. Eur J Pediatr 181, 3715–3724 (2022). https://doi.org/10.1007/s00431-022-04579-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04579-5