Abstract
Hepatitis E virus (HEV) is the causative agent of an acute self-limiting hepatitis in humans. In industrialized countries, autochthonous cases are linked to zoonotic transmission from domestic pigs, wild boar and red deer. The main route of human infection presumably is consumption of contaminated meat. Farmers, slaughterers and veterinarians are expected to be risk groups as they work close to potentially infected animals. In this study, we tested four Escherichia coli-expressed segments of the capsid protein (CP) of a German wild boar-derived HEV genotype 3 strain for their diagnostic value in an indirect immunoglobulin G (IgG) ELISA. In an initial validation experiment, a carboxy-terminal CP segment spanning amino acid (aa) residues 326–608 outperformed the other segments harbouring aa residues 112–608, 326–660 and 112–335. Based on this segment, an indirect ELISA for detection of anti-HEV IgG antibodies in human sera was established and validated using a commercial line immunoassay as reference assay. A total of 563 sera from forestry workers of all forestry offices of Brandenburg, eastern Germany and 301 sera of blood donors from eastern Germany were surveyed using these assays. The commercial test revealed seroprevalence rates of 11% for blood donors and 18% for forestry workers. These rates are in line with data obtained by the in-house test (12 and 21%). Hence, the in-house test performed strikingly similar to the commercial test (sensitivity 0.9318, specificity 0.9542). An initial screening of forestry worker and blood donor sera with a corresponding CP segment of the recently discovered Norway rat-associated HEV revealed several strong positive sera exclusively in the forestry worker panel. Future investigations have to prove the performance of this novel IgG ELISA in large-scale seroepidemiological studies. In addition, the observed elevated seroprevalence in a forestry worker group has to be confirmed by studies on groups of forestry workers from other regions. The epidemiological role of ratHEV in human disease should be assessed in a large-scale study of risk and non-risk groups.
Similar content being viewed by others
Introduction
Hepatitis E virus (HEV) is the causative agent of an acute, usually self-limiting hepatitis. The primary route of faecal-oral transmission is via contaminated drinking water. Hepatitis E is a major public health concern to people in developing countries in Asia and Africa that are usually affected by large outbreaks [1]. Nevertheless, there are increasing numbers of reports of sporadic autochthonous HEV infections in developed countries including the USA, Japan and different European countries [2]. The typical course of the disease is characterized by hepatomegaly, jaundice, fever, anorexia, nausea and abdominal pain. Usually, the viraemia is transient and occurs mainly during the prodromic phase, followed by faecal excretion of the virus [3]. However, in developed countries, most cases of hepatitis E are believed to have a subclinical course [4]. In general, the case fatality rate of hepatitis E is low with 1–4% but higher than that of hepatitis A [5].
HEV is the only member of the genus Hepevirus of the family Hepeviridae [6, 7]. The spherical non-enveloped virion has a diameter of approximately 27–34 nm. Using heterologously expressed virus-like particles, the capsid was demonstrated to have an icosahedral (T = 3) symmetry [8]. The viral genome is a single-stranded RNA of positive polarity and about 7.2 kb length (genotype 3i strain wbGER27 7,222 nucleotides (nt); [9]; GenBank accession number FJ705359.1). The genome is flanked by a 5′ untranslated region (UTR), capped at the 5′-end with 7-methylguanosine (m7G), and a 3′ UTR followed by a polyadenylated tail. It contains three partially overlapping open reading frames (ORF1, ORF2 and ORF3). ORF1 is the largest open reading frame of 5,112 nt encoding a polyprotein of 1,703 amino acids (aa) (wbGER27) with various enzymatic functions [10]. ORF2 of 1,983 nt encodes a capsid protein of 660 aa (wbGER27) representing the immunodominant protein mainly used for serodiagnostics [11]. The ORF3 of 369 nt encodes a small cytoskeleton associated phosphoprotein of 122 aa (wbGER27). It represents a viral accessory protein which is likely to affect the host response to infection [10].
In general, HEV can be subdivided phylogenetically into four genotypes and several subgenotypes: Genotypes 1 and 2 are believed to be present only in humans with genotype 1 found in Asia and Africa and genotype 2 in Mexico and Africa. Genotype 3 seems to have a world-wide distribution with current detection in Asia, Europe, Oceania, North and South America, whereas the occurrence of genotype 4 seems to be limited to Asian countries [12]. Interestingly, in contrast to genotypes 1 and 2, genotypes 3 and 4 represent zoonotic pathogens. Wild boar and domestic pigs are thought to be the major reservoirs [13]. There are several lines of evidence in support of this assumption. Firstly, sequence similarities of HEV strains from human patients and from swine suggested an epidemiological association [13]. Experimental infection studies revealed the susceptibility of domestic pigs for human strains as well as of primates for swine strains [13]. Further, direct molecular epidemiological evidence for food-borne HEV transmission to human confirmed the zoonotic nature of this agent and swine and sika deer as a reservoir [14, 15]. Moreover, HEV was molecularly detected in other mammals, including mongoose, cattle and sheep and serologically in several further mammals [16]. Besides genotypes 1–4, other HEV strains with limited nt sequence similarity have been found in wild boar [17, 18], rabbits [19], rats [20] and chicken [21–23]. Avian HEV strains largely differ from the mammalian strains, suggesting that they might represent a separate genus [23]. The determination of the complete genomic sequence of ratHEV suggested that these strains represent a novel genotype, however, the zoonotic potential of this virus remains unclear [24]. The recent identification of a HEV-related agent in different fish species raised major questions on the evolution and host adaptation of HEV [25].
Due to the short-termed viraemia, laboratory diagnosis of HEV infections in humans is mainly based on serological assays. Immunoglobulin (Ig) class M antibodies can be detected a few days after the onset of clinical symptoms, but usually disappear within 4–5 months. IgG-class antibodies appear several days later but remain detectable for up to 14 years [26]. Serological assays have been developed using recombinant ORF2- and ORF3-derived proteins expressed in E. coli, yeast, baculovirus-infected insect cells and mammalian cells. Alternatively, synthetic peptides or chimeric constructs harbouring multiple epitopes have been applied. These assays comprise ELISA and line immunoassay formats with several commercial tests available [11].
In Germany, a total of 910 human hepatitis E cases have been notified since the introduction of the Federal Protection against Infection Act in 2001 (Robert Koch-Institut, SurvStat, http://www.rki.de; data as of 14 September 2011). Several autochthonous human cases have been reported since 2006 [27–29]. A phylogenetic and case–control study confirmed the presence of autochthonous human infections with HEV genotype 3 [30]. Recent studies indicated a risk of chronic HEV infection during immunosuppression after solid organ transplantation or acute lymphoblastic leukaemia [31–33]. An initial molecular study in archived wild boar samples demonstrated the presence of HEV for at least 10 years in Germany [34]. Further molecular biological investigations on wild boar tissue samples confirmed a broad geographical distribution of HEV and resulted in the identification of different circulating genotype 3 subtypes [9, 34, 35]. Also, the consumption of undercooked wild boar meat has been identified as a risk factor for autochthonous HEV infections [30]. The recently detected sequence similarity of HEV sequences found in porcine livers from retail markets and in patients from the same geographical region suggested the importance of undercooked pig products in food as a source of zoonotic HEV infection for humans in Germany [36].
Here, we describe the development and validation of a novel indirect HEV genotype 3-based IgG ELISA and its application for a seroepidemiological study in forestry workers and blood donors from eastern Germany. In addition, these serum panels were investigated in parallel in an IgG ELISA with a corresponding ratHEV-derived recombinant antigen.
Materials and methods
Human serum samples
In 2008, a panel of 563 serum samples was collected from 499 male and 64 female forestry workers from ten different forestry districts in the federal state of Brandenburg, eastern Germany (Fig. 1; [37]). All participants provided informed consent. The control group comprises 301 serum samples of healthy blood donors from the blood donation unit of the Charité University Hospital Berlin. The donors are residents of the federal states Berlin and Brandenburg.
Cloning of HEV capsid protein-encoding sequences, expression and purification of the recombinant HEV capsid protein derivatives
For expression in E. coli, a modified pET-19b vector was generated by substituting the original BamHI/NcoI fragment by an oligonucleotide duplex harbouring the translation initiation codon, 10 histidine codons and a unique SpeI restriction site (Fig. 2a). The entire ORF2 and 5′ and 3′ truncated segments thereof, taken from a German wild boar-derived HEV genotype 3i [9], were amplified by RT-PCR using specific primers harbouring an XbaI restriction site overhang and inserted into the vector pCR-TOPO2.1 (Invitrogen, Darmstadt, Germany) (Fig. 2b). The inserts with the correct sequences were then subcloned into the SpeI-linearized modified pET-19b vector (Fig. 2b). In parallel, a pET-19b construct encoding aa residues 315–599 of the capsid protein of ratHEV strain R4 was generated ([20]; Johne et al., unpublished data). The entire coding sequence of the non-structural protein 1 (NS1) of West Nile virus (WNV) was amplified from cell culture supernatant of the New York flamingo isolate of 1999 (accession number AF196835). The amplification product obtained by RT-PCR using primers adding a 5′ NdeI restriction site and a 3′ BamHI restriction site was inserted into a non-modified NdeI and BamHI cleaved pET-19b vector (Johne et al., unpublished data). The different expression plasmids were retransformed for heterologous expression into E. coli strain BL21 (DE3) (Novagen Merck KGaA, Darmstadt, Germany). The synthesis of the recombinant proteins was induced by addition of 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). For determination of an expression kinetics, 50 ml cultures of the recombinant bacteria were induced at an OD600 value of 0.5. Samples of 1 ml each were drawn at time-points 0, 15, 30, 60, 90, 120, 150 min, 3, 4 h and after overnight incubation. The bacteria were pelleted and resuspended in 50 μl buffer containing 0.5 g/l SDS and 0.5% Triton X-100. The samples were mixed with 50 μl loading buffer, boiled and 5 μl were applied on an SDS–PAGE. The purification of the His-tagged capsid protein derivatives was performed with the Ni–NTA chromatography system under denaturing conditions according to the protocol of the manufacturer (Qiagen, Hilden, Germany).
Schematic presentation of the modified expression vector pET-19b (a) and the encoding sequences of the hepatitis E virus capsid protein along the genome of genotype 3i strain wbGER27 (b). The pET-19b vector carries a phage T7-derived promoter (P T7) and transcription terminator (T T7) upstream and downstream of the insertion site, respectively. The original pET-19b vector was modified by insertion of an oligonucleotide duplex harbouring a translation initiation codon, ten histidine codons (His10) and a unique SpeI restriction site into the BamHI/NcoI digested vector. Met methionine, His histidine, UTR untranslated region, nt nucleotide, aa amino acid
SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blot analysis
The synthesis and the purity of the recombinant CP derivatives were analysed by SDS-PAGE using 12.5 or 13% gels stained by Coomassie brilliant blue. For Western blot analysis, the proteins separated by SDS-PAGE were blotted onto a PVDF membrane. The membrane was blocked in 5% skim milk overnight at 4°C and incubated for 1 h at room temperature (RT) in anti-His monoclonal antibody (mAb) (Novagen, Merck KGaA, Darmstadt, Germany) diluted 1:2,500 in PBS-Tween 20. The antigen–antibody reaction was detected by adding horse-radish peroxidase (HRP) labelled anti-mouse Ig (Dako Cytomation, Glostrup, Denmark), diluted 1:2,000, visualized by adding ECL reagent and hydrogen superoxide (Amersham, GE Healthcare, UK) and documented using a VersaDoc Model MP 5000 Imaging-System (Bio-Rad, Munich, Germany).
Commercial serological assay
To validate the in-house ELISA, a commercial recomLine HEV IgG test (Mikrogen, Neuried, Germany) was used. This test is based on HEV genotype 1 and genotype 3 antigens. The assay was performed and read according to the manual of the manufacturer. In brief, 20 μl of serum was added to each test strip immersed in 2 ml dilution buffer and incubated for 1 h at RT. The test strips were washed three times. Incubation with HRP conjugate solution was carried out for 45 min at RT. After additional washing, the kit substrate solution (1.5 ml) was added. The reaction was stopped as soon as the cut-off control band was clearly visible (6–10 min). Interpretation of test results was performed densitometrically by means of the recomScan software. The final test result (neg., borderline, pos.) was reported based on the sum of predefined ‘points’ assigned to each positive test strip band.
In-house indirect IgG ELISA
In an initial setting, four different recombinant HEV GT-3 CP derivatives were tested according to a standard protocol previously established for investigations of human sera with recombinant hantavirus antigen [38]. This standard protocol was adopted for detection of anti-HEV IgG antibodies using a serum pool of five forestry workers found positive and a second serum pool of five forestry workers found negative in the reference assay. During this optimization process for all constructs, different types of microtitre plates and different concentrations of the capsid antigen as well as of the serum pools were assayed. Thereafter, further optimization was done with WB-Ctr (see Fig. 2b) alone, altering the concentrations of the secondary antibody, different incubation times for the secondary antibody as well as different bovine serum albumin (BSA) concentrations in blocking. The estimation of the best protocol was accomplished by judging the ratio of the results for the anti-HEV positive and negative serum pools. The final protocol is based on a 1 h coating at 37°C with 100 μl of 1 μg/ml protein in 0.05 M carbonate buffer (pH 9.8) per well, 1 h blocking at RT with 200 μl of PBS containing 3% BSA and 0.05% Tween 20, 1 h incubation at 37°C with 100 μl serum diluted 1:400 in PBS with 1% BSA and 0.05% Tween 20, and a final 1 h incubation at 37°C with 100 μl of HRP-conjugated anti-human IgG (rabbit polyclonal, P0214, Dako, Glostrup, Denmark) diluted 1:6,000 in PBS with 1% BSA and 0.05% Tween 20. The enzyme reaction was performed using 100 μl 3,3′,5,5′-Tetramethylbenzidine (Peroxidase EIA Substrate Kit, Bio-Rad, Hercules, CA, USA) as substrate, stopped by adding 100 μl 1 M H2SO4 and visualized at 450 nm. The same protocol was used for the ratHEV-derived antigen and the negative control antigen.
Determination of the cut-off value for the in-house indirect IgG ELISA
For the evaluation of the ELISA, we used the commercial line assay as a reference method. To find a variable, plate-dependent cut-off value based on the positive and negative control serum pools, a deviation of the receiver operating characteristic (ROC) curve was evaluated by several methods. To this end, cut-off values being defined as percentage of the positive control, of the negative control with or without the blank value subtracted, a summand added to the negative control with or without the blank value subtracted and the cut-off of the index [(reading-neg)/(pos-neg)] were tested. The optimal method of cut-off calculation was determined by the area under curve (AUC) value. The optimal cut-off value was selected based on the minimum ROC distance, i.e. the distance between the curve and the upper left corner of the graph.
Statistical analysis
The statistical significance of the difference of the antigenic efficacy as ELISA coat as well as the significance regarding sex and risk-group/control-group affiliation was examined using a Wilcoxon rank sum test with continuity correction. The analysis of the ELISA results from the different forestry districts were done with a two-sided Fisher’s Exact Test. All calculations were performed using R, version 2.13.0 (2011-04-13) [39].
Results
Expression, purification and characterization of HEV capsid protein derivatives
An initial approach to express the entire ORF2 of HEV genotype 3i strain wbGER27 failed. The expression of the entire ORF2 was neither detected in crude lysates in the stained SDS-PAGE nor by Western blot analysis using the His-tag-specific mAb independently of the induction time. In addition, a nickel chelate affinity purification approach also failed to enrich the entire capsid protein (data not shown). Therefore, four truncated, partially overlapping segments of the ORF2-encoding region were generated (Fig. 2b). The analysis of total lysates from E. coli transformed with the four recombinant plasmids revealed the synthesis of the CP derivatives WB-tr, WB-Ntr, WB-C and WB-Ctr (see Fig. 2b) corresponding to their respective molecular weights. A subsequent Western blot analysis of these four crude lysates with a His-tag-specific mAb confirmed the authenticity of these recombinant proteins with an amino-terminal His-tag (data not shown). The main peak of protein synthesis was detected by an expression kinetics approach after a 3 h induction with IPTG for all four proteins (data not shown). These recombinant proteins were found to be insoluble in inclusion bodies, but were solubilized in 8 M urea prior to purification. Separating the inclusion bodies from the soluble proteins by centrifugation followed by a single step of nickel chelate affinity chromatography and elution in a low pH buffer and imidazole resulted in highly purified proteins of the expected molecular weights (Fig. 3).
Analysis of the purified E. coli-expressed capsid protein derivatives, the truncated almost complete capsid protein (WB-tr), the truncated amino-terminal part of capsid protein (WB-Ntr), and the carboxy-terminal part (WB-C) and truncated carboxy-terminal part (WB-Ctr) of the capsid protein of genotype 3i strain wbGER27 in a Coomassie blue-stained 13% SDS–polyacrylamide gel. The West Nile virus (WNV), strain New York, non-structural protein 1 (NS1) expressed in the same heterologous system and purified in the same way was used as a control antigen. M, molecular weight marker (PageRuler unstained SM0661, Fermentas). The molecular weights given above the recombinant protein bands were predicted using a web-based calculator (http://www.bioinformatics.org/sms/prot_mw.html)
ELISA development and validation
To identify the most useful recombinant antigen for serodiagnostics, all four purified antigens and a negative control antigen, i.e. WNV NS1 protein, were tested for their reactivity with an anti-HEV positive and a negative serum pool. As expected, the ratio of the OD values for the positive and negative serum pool was close to 1 for the negative control antigen (Fig. 4). The truncated amino-terminal CP derivative behaved similarly, i.e. it was not able to discriminate between the positive and negative pools. The almost entire CP (WB-tr) and the carboxy-terminal derivatives (WB-C and WB-Ctr) performed better with significant differences to the negative control antigen and to each other (Fig. 4). The WB-Ctr antigen demonstrated an average ratio of 10.6 (range 8.1–14.1) of the OD values for the positive and the negative serum pool and was therefore selected for subsequent investigations.
Comparison of the reactivity of the purified capsid protein derivatives with anti-HEV positive and negative human serum pools in ELISA. Five sera each found to be negative or positive in the reference assay were pooled and used for analysis in the indirect ELISA using the truncated almost complete capsid protein (WB-tr), the truncated amino-terminal part of capsid protein (WB-Ntr), and the carboxy-terminal part (WB-C) and truncated carboxy-terminal part (WB-Ctr) of the capsid protein of genotype 3i strain wbGER27. For determination of the antigen with the strongest specific reactivity, the ratio of the corrected OD values of the positive pool and the negative pool was calculated. The columns show the mean value and standard error of the mean for five replicates. Statistically significant correlations are marked with an asterisk (P < 0.01)
To determine the performance of the novel in-house IgG ELISA, 555 serum samples from forestry workers and 298 serum samples from blood donors were screened in parallel by this assay and the commercial recomLine HEV IgG assay serving as reference test. For the majority of anti-HEV-positive and -negative forestry worker (Table 1) and blood donor sera (Table 2), the results were concordant. A modified ROC curve analysis using seven different approaches resulted in AUC values of 0.9774–0.9499 and corresponding sensitivities of 0.9167–0.9318 and specificities of 0.9667–0.9376 (Fig. 5). Based on the method yielding the highest AUC value, the variable cut-off value was defined at 5.425% of the difference of the positive control and the negative control resulting in a sensitivity of 0.9318 and a specificity of 0.9542 (Fig. 5a).
Determination of the optimal variable cut-off value for the genotype 3i wbGER27 capsid protein derivative based indirect ELISA by a modified receiver operating characteristic (ROC) analysis using three different approaches. The graphs show the performance of the novel in-house test (and its sensitivities and specificities at the cut-off of the minimal ROC distance) when the cut-off value is defined as percentage of the difference of the positive control and the negative control (a), as the percentage of the positive control (b) and by finding a factor of the negative control (c). The selection of the optimal cut-off value for the further investigations (as given in a) was based on the highest area under curve (AUC) value. pos positive, neg negative, fact factor
HEV seroprevalence of the forestry worker and blood donor panels
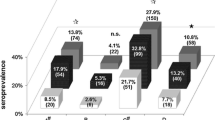
The average seroprevalence for the forestry workers reached 17.8 and 21.4% when using the commercial and the in-house test, respectively. A more precise analysis demonstrated that in all ten forestry districts anti-HEV positive individuals were detected, independently of the test used (Table 3). The prevalences for the forestry districts ranged from 5.6% (Doberlug-Kirchhain, recomLine) and 9.1% (Peitz, in-house test) to 25% (Alt Ruppin, both tests), 26.8 and 28% (Belzig and Wünsdorf, in-house test).
When compared to the forestry worker panel, for the blood donor panel lower seroprevalences of 11.1% (recomLine) and 12.3% (in-house test) were determined. The seroprevalences for male blood donors were almost identical for both assays (12.1% vs. 12.9%), whereas the values for female blood donors varied between 7.5% for the recomLine assay and 10.3% for the in-house ELISA. Independently from the test used, the seroprevalence for male subjects was slightly higher than for female subjects, but not of statistical significance in both tests and with all populations (blood donors, forestry workers or both). The analysis of age and anti-HEV prevalence revealed no significant association of these two factors, regardless of the test and of the population viewed (blood donors, forestry workers or both) (Fig. 6).
Ratios of anti-HEV-positive and anti-HEV-negative male forestry workers (a, e) and female forestry workers (b, f) and anti-HEV-positive and anti-HEV-negative male blood donors (c, g) and anti-HEV-positive and anti-HEV-negative female blood donors (d, h) according to age based on the analyses with the recomLine reference assay (a–d) and the novel in-house assay (e–h). White segments of the columns represent seronegative subjects, black segments represent seropositive subjects
Reactivity of the forestry worker and blood donor serum panels with a recombinant ratHEV antigen
In addition to the IgG ELISA analysis of the serum panels with the genotype 3i HEV antigen, the sera were screened in parallel with the corresponding ratHEV-derived antigen. This antigen was found to be expressed in E. coli at high level and could be readily purified in the same manner as the genotype 3i antigen (Johne et al., unpublished data). Interestingly, in the forestry worker panel, several sera with an almost exclusive and relatively strong reactivity with the ratHEV CP derivative were observed (Fig. 7a). On the other hand, only a very few of the blood donor sera were found to be very weakly reactive with the ratHEV antigen (Fig. 7b).
Reactivity of forestry worker (a) and blood donor sera (b) with corresponding recombinant capsid protein derivatives of genotype 3i (WB-Ctr, Y-axis) and ratHEV (R-Ctr, X-axis) in the ELISA. For each serum sample, a parallel analysis in both ELISAs was performed. The values depicted represent percentage values of the corrected OD values in relation to the positive anti-HEV-GT3i and anti-ratHEV control set to 100%. The black vertical line denotes the percentage (26.0%) of the blood donor sample with the strongest reactivity with ratHEV antigen
Discussion
Here, we report on the diagnostic value of different segments of the CP of HEV genotype 3i. Due to failure in expression of the entire ORF2 in the E. coli expression system, carboxy- and amino-terminal segments of the CP were generated in E. coli. In line with previous investigations [40–42], the carboxy-terminal segments spanning aa residues 326–608 and 326–660 were found to be highly reactive with a pool of anti-HEV positive human sera. Additionally, immunodominant antigenic regions were previously found in this region using peptide-based assays [43, 44]. The observation of the strong antigenicity of the carboxy-terminal region is also in line with the localization of the protruding domain in the CP structure between aa 456 and aa 606 [45] and the localization of the immunodominant viral-neutralizing epitope inside this part of CP [46]. Peptide scanning demonstrated a large number of linear epitopes in the carboxy-terminal region, but linear epitopes were found to be scattered throughout the entire protein [47]. However, the amino-terminal segment harbouring aa 112–335 was not able to discriminate between the seropositive and seronegative pools.
Therefore, an indirect IgG ELISA was developed based on the most reactive carboxy-terminal segment. When testing two different serum panels, a panel of forestry workers and a panel of blood donors, the performance of the novel in-house assay was similar to that of the commercial reference test used. Based on the ROC-mediated definition of a cut-off value, the sensitivity and specificity of the novel assay was determined to be 0.9318 and 0.9542, respectively. Thereby, this novel genotype 3-based IgG ELISA performs comparably to other serological assays developed using E. coli-expressed [48] or baculovirus-expressed ORF2 antigens [49, 50].
The seroprevalences of 11.1 and 12.3% observed in the blood donor panel were surprisingly high, but seem to be in the same range as recent studies found in Germany [51, 52, 66]. The similar values obtained for this panel using two different assays might clearly argue against a large number of false-positive sera, as discussed recently [11]. Similarly, high seroprevalences in the average population were previously reported for other non-epidemic regions in Europe, i.e. in Denmark with 20.6% [53], and the UK with 16% [54]. However, for some non-epidemic countries, i.e. the Netherlands (1.1%; [55]), Italy (1.0%; [56]), Switzerland (3.2%; [57]), Spain (2.8%; [58]), USA (1.1%; [59]) and Japan (5.3%; [60]) rather low prevalences were reported. These conflicting findings underscore the need for developing standardized serological assays for comparative analyses of HEV seroprevalences. The rather high seroprevalences found in this study on one hand and the low number of notified hepatitis E cases in Germany on the other might indicate a high number of infections with mild or unspecific symptoms that are therefore not recorded. In addition, this finding raised important questions on the transmission route of HEV genotype 3. Therefore, additional epidemiological studies have to prove the role of transmission routes other than oral uptake of contaminated food, blood transfusion and organ transplantation.
The seroprevalence in the forestry worker group (18 and 21%) was found higher than that of the blood donor control group (11 and 12%). This difference was found to be significant (P < 0.01), but both groups are not sufficiently matching in sex, age and residence (see Fig. 6). Nevertheless, these findings still confirm a HEV seroprevalence in the investigated part of Germany of more than 10%. In the past, forestry workers have not been considered as a typical risk group for HEV infection. However, hunting activities are usually included in the professional work of forestry workers, including the group investigated in this study. This risk might be especially taken into account if the HEV prevalence in the wild boar population at site is high, as previously reported for Brandenburg [9, 35]. These findings may also underline other transmission routes for HEV such as direct contact to wild boar blood or faeces or their aerosols. Another recent study in Germany has reported an increased HEV seroprevalence in slaughterers [52]. Studies in other European countries and the USA have also demonstrated increased HEV seroprevalences in farmers and veterinarians exposed to swine (for references see [53, 61, 62]). On the other hand, other investigations did not find differences in seroprevalence between risk and non-risk groups [63, 64] emphasizing the above mentioned need for test harmonization. Recently, a scoping study that used systematic review/meta-analysis methodology confirmed a significant association between occupational exposure to swine and human HEV IgG seropositivity in 10 out of 13 cross-sectional studies [65]. Interestingly, a recent study demonstrated a HEV seroprevalence of 30% in psychiatric patients that was ascribed to patients of addiction therapy, including alcohol, benzodiazepine and intravenous drug addicts [66].
In our study, we did not find a statistically significant association of seropositivity with age and sex in neither group and independently of the test used. Until now there are controversial findings on the influence of sex and age on the level of the seroprevalence [2]. Thus, a recent study found in a group older than 40 years a significantly higher prevalence than in a group younger than 14 years, but no significant differences when analysing age groups step by step [66]. Interestingly, 62.5% of the notified hepatitis E cases in Germany were found in men (Robert Koch-Institut, SurvStat, http://www.rki.de; data as of 14 September 2011). Future studies should prove if sex-hormone-mediated factors or alcohol abuse may influence the outcome of HEV infections.
A parallel analysis of forestry worker and blood donor sera in the genotype 3i- and a similar ratHEV-IgG ELISA suggested for the first time the occurrence of human infections by ratHEV or a related agent in a few cases. The two recombinant antigens used for this serological investigation shared a total aa sequence identity of 54% with an identity of 45% in the protruding domain [45] indicating antigenic differences in the CP of genotype 3 and ratHEV. Recent investigations of hyperimmune sera from rats demonstrated clear antigenic differences between both genotypes, at least for the antigen segment used (Johne et al., unpublished data). Cross-protection studies will help to determine whether ratHEV belongs to the same single serotype with the four major genotypes of HEV. Future studies in risk groups, such as pest management or sewage workers, and control groups have to further elaborate the potential of ratHEV to cause human infections and disease. In addition, this finding again raises the question of the transmission route of ratHEV or a related virus to humans.
In conclusion, a novel genotype 3-based ELISA was developed and showed similar sensitivity and specificity as compared to a commercial reference test. Application of the novel assay and the reference test resulted in the detection of HEV-specific antibodies in blood donors and forestry workers from eastern Germany with an unexpected high frequency. An initial analysis of both groups with a similar ratHEV-based ELISA demonstrated for the first time the occurrence of human infections. Future larger seroepidemiological studies in non-risk and risk groups should further investigate the epidemiological importance of ratHEV.
References
Aggarwal R (2011) Hepatitis E: historical, contemporary and future perspectives. J Gastroenterol Hepatol 26(Suppl 1):72–82
Lewis HC, Wichmann O, Duizer E (2010) Transmission routes and risk factors for autochthonous hepatitis E virus infection in Europe: a systematic review. Epidemiol Infect 138(2):145–166
Pavio N, Meng XJ, Renou C (2010) Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res 41(6):46
Dalton HR, Bendall R, Ijaz S, Banks M (2008) Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis 8(11):698–709
Purcell RH, Emerson SU (2008) Hepatitis E: an emerging awareness of an old disease. J Hepatol 48(3):494–503
Emerson S, Anderson D, Arankalle A, Meng X, Purdy M, Schlauder G (2004) Hepevirus. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Virus taxonomy. VIIIth report of the ICTV. Elsevier/Academic Press, London, pp 851–855
International Committee on Taxonomy of Viruses (2009) http://www.ictvonline.org/virusTaxonomy.asp?version=2009
Xing L, Li TC, Mayazaki N, Simon MN, Wall JS, Moore M, Wang CY, Takeda N, Wakita T, Miyamura T, Cheng RH (2010) Structure of hepatitis E virion-sized particle reveals an RNA-dependent viral assembly pathway. J Biol Chem 285(43):33,175–33183
Schielke A, Sachs K, Lierz M, Appel B, Jansen A, Johne R (2009) Detection of hepatitis E virus in wild boars of rural and urban regions in Germany and whole genome characterization of an endemic strain. Virol J 6:58
Ahmad I, Holla RP, Jameel S (2011) Molecular virology of hepatitis E virus. Virus Res 161(1):47–58
Khudyakov Y, Kamili S (2011) Serological diagnostics of hepatitis E virus infection. Virus Res 161(1):84–92
Lu L, Li C, Hagedorn CH (2006) Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol 16(1):5–36
Meng XJ (2010) Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol 140(3–4):256–265
Tei S, Kitajima N, Takahashi K, Mishiro S (2003) Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362(9381):371–373
Tei S, Kitajima N, Ohara S, Inoue Y, Miki M, Yamatani T, Yamabe H, Mishiro S, Kinoshita Y (2004) Consumption of uncooked deer meat as a risk factor for hepatitis E virus infection: an age- and sex-matched case-control study. J Med Virol 74(1):67–70
Meng XJ (2011) From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res 161(1):23–30
Sato Y, Sato H, Naka K, Furuya S, Tsukiji H, Kitagawa K, Sonoda Y, Usui T, Sakamoto H, Yoshino S, Shimizu Y, Takahashi M, Nagashima S, Jirintai, Nishizawa T, Okamoto H (2011) A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch Virol 156(8):1345–1358
Takahashi M, Nishizawa T, Sato H, Sato Y, Jirintai, Nagashima S, Okamoto H (2011) Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J Gen Virol 92(Pt 4):902–908
Zhao C, Ma Z, Harrison TJ, Feng R, Zhang C, Qiao Z, Fan J, Ma H, Li M, Song A, Wang Y (2009) A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J Med Virol 81(8):1371–1379
Johne R, Plenge-Bönig A, Hess M, Ulrich RG, Reetz J, Schielke A (2010) Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J Gen Virol 91(Pt 3):750–758
Payne CJ, Ellis TM, Plant SL, Gregory AR, Wilcox GE (1999) Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet Microbiol 68(1–2):119–125
Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ (2001) Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J Gen Virol 82(Pt 10):2449–2462
Marek A, Bilic I, Prokofieva I, Hess M (2010) Phylogenetic analysis of avian hepatitis E virus samples from European and Australian chicken flocks supports the existence of a different genus within the Hepeviridae comprising at least three different genotypes. Vet Microbiol 145(1–2):54–61
Johne R, Heckel G, Plenge-Bönig A, Kindler E, Maresch C, Reetz J, Schielke A, Ulrich RG (2010) Novel hepatitis E virus genotype in Norway rats, Germany. Emerg Infect Dis 16(9):1452–1455
Batts W, Yun S, Hedrick R, Winton J (2011) A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii). Virus Res 158(1–2):116–123
Aggarwal R, Krawczynski K (2000) Hepatitis E: an overview and recent advances in clinical and laboratory research. J Gastroenterol Hepatol 15(1):9–20
Preiss JC, Plentz A, Engelmann E, Schneider T, Jilg W, Zeitz M, Duchmann R (2006) Autochthonous hepatitis E virus infection in Germany with sequence similarities to other European isolates. Infection 34(3):173–175
Brost S, Wenzel JJ, Ganten TM, Filser M, Flechtenmacher C, Boehm S, Astani A, Jilg W, Zeier M, Schnitzler P (2010) Sporadic cases of acute autochthonous hepatitis E virus infection in Southwest Germany. J Clin Virol 47(1):89–92
Veitt R, Reichardt M, Wenzel J, Jilg W (2011) Autochthonous hepatitis E-virus infection as cause of acute hepatitis in Germany—a case report. Z Gastroenterol 49(1):42–46
Wichmann O, Schimanski S, Koch J, Kohler M, Rothe C, Plentz A, Jilg W, Stark K (2008) Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis 198(12):1732–1741
le Coutre P, Meisel H, Hofmann J, Röcken C, Vuong GL, Neuburger S, Hemmati PG, Dörken B, Arnold R (2009) Reactivation of hepatitis E infection in a patient with acute lymphoblastic leukaemia after allogeneic stem cell transplantation. Gut 58(5):699–702
Pischke S, Suneetha PV, Baechlein C, Barg-Hock H, Heim A, Kamar N, Schlue J, Strassburg CP, Lehner F, Raupach R, Bremer B, Magerstedt P, Cornberg M, Seehusen F, Baumgaertner W, Klempnauer J, Izopet J, Manns MP, Grummer B, Wedemeyer H (2010) Hepatitis E virus infection as a cause of graft hepatitis in liver transplant recipients. Liver Transpl 16(1):74–82
Schlosser B, Stein A, Neuhaus R, Pahl S, Ramez B, Krüger DH, Berg T, Hofmann J (2011) Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol (in press)
Kaci S, Nöckler K, Johne R (2008) Detection of hepatitis E virus in archived German wild boar serum samples. Vet Microbiol 128(3–4):380–385
Adlhoch C, Wolf A, Meisel H, Kaiser M, Ellerbrok H, Pauli G (2009) High HEV presence in four different wild boar populations in East and West Germany. Vet Microbiol 139(3–4):270–278
Wenzel JJ, Preiss J, Schemmerer M, Huber B, Plentz A, Jilg W (2011) Detection of hepatitis E virus (HEV) from porcine livers in Southeastern Germany and high sequence homology to human HEV isolates. J Clin Virol 52(1):50–54
Mertens M, Hofmann J, Petraityte-Burneikiene R, Ziller M, Sasnauskas K, Friedrich R, Niederstrasser O, Krüger DH, Groschup MH, Petri E, Werdermann S, Ulrich RG (2011) Seroprevalence study in forestry workers of a non-endemic region in eastern Germany reveals infections by Tula and Dobrava-Belgrade hantaviruses. Med Microbiol Immunol 200(4):263–268
Mertens M, Wölfel R, Ullrich K, Yoshimatsu K, Blumhardt J, Römer I, Esser J, Schmidt-Chanasit J, Groschup MH, Dobler G, Essbauer SS, Ulrich RG (2009) Seroepidemiological study in a Puumala virus outbreak area in South-East Germany. Med Microbiol Immunol 198(2):83–91
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Obriadina A, Meng JH, Ulanova T, Trinta K, Burkov A, Fields HA, Khudyakov YE (2002) A new enzyme immunoassay for the detection of antibody to hepatitis E virus. J Gastroenterol Hepatol 17(Suppl 3):S360–S364
Chen HY, Lu Y, Howard T, Anderson D, Fong PY, Hu WP, Chia CP, Guan M (2005) Comparison of a new immunochromatographic test to enzyme-linked immunosorbent assay for rapid detection of immunoglobulin M antibodies to hepatitis E virus in human sera. Clin Diagn Lab Immunol 12(5):593–598
Hu WP, Lu Y, Precioso NA, Chen HY, Howard T, Anderson D, Guan M (2008) Double-antigen enzyme-linked immunosorbent assay for detection of hepatitis E virus-specific antibodies in human or swine sera. Clin Vaccine Immunol 15(8):1151–1157
Khudyakov YE, Favorov MO, Khudyakova NS, Cong ME, Holloway BP, Padhye N, Lambert SB, Jue DL, Fields HA (1994) Artificial mosaic protein containing antigenic epitopes of hepatitis E virus. J Virol 68(11):7067–7074
Riddell MA, Li F, Anderson DA (2000) Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies. J Virol 74(17):8011–8017
Yamashita T, Mori Y, Miyazaki N, Cheng RH, Yoshimura M, Unno H, Shima R, Moriishi K, Tsukihara T, Li TC, Takeda N, Miyamura T, Matsuura Y (2009) Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc Natl Acad Sci USA 106(31):12,986–12991
Li F, Riddell MA, Seow HF, Takeda N, Miyamura T, Anderson DA (2000) Recombinant subunit ORF2.1 antigen and induction of antibody against immunodominant epitopes in the hepatitis E virus capsid protein. J Med Virol 60(4):379–386
Khudyakov YE, Lopareva EN, Jue DL, Crews TK, Thyagarajan SP, Fields HA (1999) Antigenic domains of the open reading frame 2-encoded protein of hepatitis E virus. J Clin Microbiol 37(9):2863–2871
Zhou YH, Purcell RH, Emerson SU (2004) An ELISA for putative neutralizing antibodies to hepatitis E virus detects antibodies to genotypes 1, 2, 3, and 4. Vaccine 22(20):2578–2585
Arankalle VA, Lole KS, Deshmukh TM, Chobe LP, Gandhe SS (2007) Evaluation of human (genotype 1) and swine (genotype 4)-ORF2-based ELISAs for anti-HEV IgM and IgG detection in an endemic country and search for type 4 human HEV infections. J Viral Hepat 14(6):435–445
de Oya J, Galindo I, Gironés O, Duizer E, Escribano JM, Saiz JC (2009) Serological immunoassay for detection of hepatitis E virus on the basis of genotype 3 open reading frame 2 recombinant proteins produced in Trichoplusia in larvae. J Clin Microbiol 47(10):3276–3282
Meisel H (2009) Hepatitis-E-Virus. In: Neumeister B, Braun R, Kimmig P (eds) Mikrobiologische Diagnostik. Georg Thieme Verlag, Stuttgart, pp 856–861
Krumbholz A, Mohn U, Lange J, Motz M, Wenzel JJ, Jilg W, Walther M, Straube E, Wutzler P, Zell R (2011) Prevalence of hepatitis E virus-specific antibodies in humans with occupational exposure to pigs. Med Microbiol Immunol (in press)
Christensen PB, Engle RE, Hjort C, Homburg KM, Vach W, Georgsen J, Purcell RH (2008) Time trend of the prevalence of hepatitis E antibodies among farmers and blood donors: a potential zoonosis in Denmark. Clin Infect Dis 47(8):1026–1031
Dalton HR, Stableforth W, Thurairajah P, Hazeldine S, Remnarace R, Usama W, Farrington L, Hamad N, Sieberhagen C, Ellis V, Mitchell J, Hussaini SH, Banks M, Ijaz S, Bendall RP (2008) Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol 20(8):784–790
Zaaijer HL, Kok M, Lelie PN, Timmerman RJ, Chau K, van der Pal HJ (1993) Hepatitis E in The Netherlands: imported and endemic. Lancet 341(8848):826
Zanetti AR, Dawson GJ (1994) Hepatitis type E in Italy: a seroepidemiological survey. Study group of hepatitis E. J Med Virol 42(3):318–320
Lavanchy D, Morel B, Frei PC (1994) Seroprevalence of hepatitis E virus in Switzerland. Lancet 344(8924):747–748
Mateos ML, Camarero C, Lasa E, Teruel JL, Mir N, Baquero F (1998) Hepatitis E virus: relevance in blood donors and other risk groups. Vox Sang 75(4):267–269
Eick A, Ticehurst J, Tobler S, Nevin R, Lindler L, Hu Z, MacIntosh V, Jarman R, Gibbons R, Myint K, Gaydos J (2010) Hepatitis E seroprevalence and seroconversion among US military service members deployed to Afghanistan. J Infect Dis 202(9):1302–1308
Takahashi M, Tamura K, Hoshino Y, Nagashima S, Yazaki Y, Mizuo H, Iwamoto S, Okayama M, Nakamura Y, Kajii E, Okamoto H (2010) A nationwide survey of hepatitis E virus infection in the general population of Japan. J Med Virol 82(2):271–281
Withers MR, Correa MT, Morrow M, Stebbins ME, Seriwatana J, Webster WD, Boak MB, Vaughn DW (2002) Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am J Trop Med Hyg 66(4):384–388
Meader E, Thomas D, Salmon R, Sillis M (2010) Seroprevalence of hepatitis E virus in the UK farming population. Zoonoses Public Health 57(7–8):504–509
Vulcano A, Angelucci M, Candelori E, Martini V, Patti AM, Mancini C, Santi AL, Calvani A, Casagni L, Lamberti A (2007) HEV prevalence in the general population and among workers at zoonotic risk in Latium Region. Ann Ig 19(3):181–186
Olsen B, Axelsson-Olsson D, Thelin A, Weiland O (2006) Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scand J Infect Dis 38(1):55–58
Wilhelm BJ, Rajic A, Greig J, Waddell L, Trottier G, Houde A, Harris J, Borden LN, Price C (2011) A systematic review/meta-analysis of primary research investigating swine, pork or pork products as a source of zoonotic hepatitis E virus. Epidemiol Infect 139(8):1127–1144
Reinheimer C, Allwinn R, Berger A (2011) Hepatitis E: are psychiatric patients on special risk? Med Microbiol Immunol (in press)
Acknowledgments
We would like to thank Alexandrina Schmidt, Ute Wessels, Thomas Büchner, Maya Gussmann, Monika Ehrl and Bianka Ehrlich for excellent technical assistance. The purified recombinant WNV NS1 protein was kindly provided by Katja Schmidt. This work was funded in part by the German Federal Ministry of Food, Agriculture and Consumer Protection (BMELV) through the Federal Institute for Agriculture and Nutrition (BLE), grant numbers 07HS026 and 07HS027 (Contract No.: 506122) and the Grimminger-Stiftung. PD acknowledges support by the Förderverein of the Friedrich-Loeffler-Institut.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dremsek, P., Wenzel, J.J., Johne, R. et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med Microbiol Immunol 201, 189–200 (2012). https://doi.org/10.1007/s00430-011-0221-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-011-0221-2