Abstract
Tinnitus is one of the main hearing impairments often associated with pure-tone hearing loss, and typically manifested in the perception of phantom sounds. Nevertheless, tinnitus has traditionally been studied in isolation without necessarily considering auditory ghosting and hearing loss as part of the same syndrome. Hence, in the present neuroanatomical study, we attempted to pave the way toward a better understanding of the tinnitus syndrome, and compared two groups of almost perfectly matched individuals with (TIHL) and without (NTHL) pure-tone tinnitus, but both characterized by pure-tone hearing loss. The two groups were homogenized in terms of sample size, age, gender, handedness, education, and hearing loss. Furthermore, since the assessment of pure-tone hearing thresholds alone is not sufficient to describe the full spectrum of hearing abilities, the two groups were also harmonized for supra-threshold hearing estimates which were collected using temporal compression, frequency selectivity und speech-in-noise tasks. Regions-of-interest (ROI) analyses based on key brain structures identified in previous neuroimaging studies showed that the TIHL group exhibited increased cortical volume (CV) and surface area (CSA) of the right supramarginal gyrus and posterior planum temporale (PT) as well as CSA of the left middle-anterior part of the superior temporal sulcus (STS). The TIHL group also demonstrated larger volumes of the left amygdala and of the left head and body of the hippocampus. Notably, vertex-wise multiple linear regression analyses additionally brought to light that CSA of a specific cluster, which was located in the left middle-anterior part of the STS and overlapped with the one found to be significant in the between-group analyses, was positively associated with tinnitus distress level. Furthermore, distress also positively correlated with CSA of gray matter vertices in the right dorsal prefrontal cortex and the right posterior STS, whereas tinnitus duration was positively associated with CSA and CV of the right angular gyrus (AG) and posterior part of the STS. These results provide new insights into the critical gray matter architecture of the tinnitus syndrome matrix responsible for the emergence, maintenance and distress of auditory phantom sensations.
Similar content being viewed by others
Introduction
Tinnitus is commonly experienced as ringing, hissing or noise in the ears in the absence of any corresponding physical sound source in the environment (Baguley et al. 2013; Cederroth et al. 2019). Such phantom auditory sensations are one of the main otological symptoms frequently associated with pure-tone hearing loss (Axelsson and Ringdahl 1989; Savastano 2008), have an estimated lifetime prevalence in the range of 9–26% (McCormack et al. 2014; Sahlsten et al. 2018), and are supposed to increase in parallel with age-related hearing impairments (Oosterloo et al. 2021; Rosing et al. 2016). Tinnitus is usually characterized by a chronic (> 12 month) course, can be extremely disturbing and bothersome, and is, therefore, often accompanied by psychological distress (Adjamian et al. 2014; Axelsson and Ringdahl 1989; Savastano 2008). Nevertheless, there is reason to believe that the latter discomfort tends to improve over time without mandatory changes in phantom sound characteristics (Vielsmeier et al. 2020; Wallhausser-Franke et al. 2017).
Although the etiology of tinnitus is multifactorial in nature and its pathophysiology remains somewhat elusive (Adjamian et al. 2014), the frequent co-occurrence (~ 90%) of tinnitus and pure-tone hearing loss (Davis and El Rafaie 2000) has led to the assumption that both disorders are anchored to a common origin in the inner ear (Gu et al. 2010). According to this perspective, damage to inner hair cells of the cochlea is supposed to affect neural impulse transmission along the peripheral auditory pathway, and to promote thalamocortical dysrhythmia (Llinas et al. 1999; Meyer et al. 2014). More specifically, as a consequence of deafferentiation following hair cell loss in the cochlea, the auditory thalamus starts generating spontaneous low-frequency oscillations which result in hyperactivity of auditory cortical fields (Llinas et al. 1999). A complementary neural mechanism which has been proposed to contribute to the emergence and maintenance of tinnitus is a dysbalance between excitation and inhibition in the auditory cortex, possibly due to bottom-up input deprivation (Eggermont 2007, 2008; Okamoto et al. 2010). Thereby, deafferentiated neurons in the auditory cortex become sensitive to adjacent frequencies, lose their lateral inhibition properties, and the resulting maladaptive reorganization of tonotopic maps leads to inherent rhythmic oscillations which are manifested in an over-representation of the tinnitus frequency (Eggermont 2007, 2008).
The view that tinnitus is primarily determined by cochlear dysfunctions (Schmidt et al. 2018) has been challenged by single-case reports showing that a dissection of the auditory nerve was not necessarily accompanied by a relief of the symptoms (House and Brackmann 1981). In addition, the absence of measurable pure-tone hearing loss in some individuals suffering from tinnitus raises further questions about the generalizability of the neural principles essentially involved in auditory phantom sensations (Weisz et al. 2006). Hence, tinnitus cannot be attributed exclusively to a dysfunction of the inner ear or to maladaptive plasticity in the auditory cortex, but should rather be considered as a syndrome involving large-scale neural networks and multiple structures of the central nervous system (De Ridder et al. 2011). The plausibility of this argument is strengthened by functional neuroimaging studies showing that tinnitus is associated with aberrant brain activity patterns in cortical–subcortical circuits including both auditory and non-auditory brain regions (Kleinjung and Langguth 2020). While some studies provided evidence for tinnitus-related functional peculiarities in the inferior colliculi, the thalamus, and the auditory cortex (Arnold et al. 1996; Eichhammer et al. 2003; Giraud et al. 1999; Langguth et al. 2006; Lanting et al. 2008; Melcher et al. 2000; Smits et al. 2007), other reported dysfunctional brain activity in limbic regions, the inferior parietal lobe, the anterior cingulate cortex as well as in different subdivision of the prefrontal cortex (Araneda et al. 2018; Carpenter-Thompson et al. 2015; Cheng et al. 2020; Golm et al. 2013; Lanting et al. 2009; Seydell-Greenwald et al. 2012). Drawing on this background, it is conceivable that pathophysiological mechanisms in peripheral auditory structures constitute a critical step in the development of tinnitus, while the consequent altered signal transmission to the central auditory system as well as maladaptive plastic changes in several cortical brain areas are responsible for its manifestation and maintenance (Lenarz et al. 1993).
Since brain functions are determined, among other factors, by the underlying neural architecture (Batista-Garcia-Ramo and Fernandez-Verdecia 2018; Lehericy et al. 2000), in the last decade, more and more studies began to examine tinnitus-related gray matter changes using voxel-based (VBM) or surface-based morphometry (SBM) (Allan et al. 2016; Liu et al. 2018; Meyer et al. 2016; Schecklmann et al. 2013; Scott-Wittenborn et al. 2017; Wei et al. 2021; Muhlau et al. 2006), whereas the microstructural properties of white matter pathways have commonly been estimated by means of diffusion tensor imaging (DTI) techniques (Benson et al. 2014; Husain et al. 2011; Schmidt et al. 2018). Currently, two main avenues of research have been pursued to characterize the neuroanatomical hallmarks of tinnitus. A first approach relies on the comparison between individuals with and without tinnitus who demonstrate pure-tone audiometric profiles in the normative range (pure-tone thresholds < 25 dB hearing loss) (Aldhafeeri et al. 2012; Besteher et al. 2019; Landgrebe et al. 2009; Muhlau et al. 2006; Schmidt et al. 2018). Although such a procedure is particularly fruitful to control for the influence of hearing acuity, it does not take into account the high comorbidity of tinnitus and pure-tone hearing loss (Davis and El Rafaie 2000). Therefore, a second alternative strategy consists of considering both auditory-related disorders as a single entity rather than as separate or confounding parts (Allan et al. 2016; Benson et al. 2014; Schneider et al. 2009; Vanneste et al. 2015). In this sense, the nexus between tinnitus and pure-tone hearing loss can be addressed, for example, by comparing two groups of individuals with and without phantom manifestations but both exhibiting pure-tone hearing loss (Allan et al. 2016; Benson et al. 2014; Vanneste et al. 2015).
The first experimental approach aiming at mapping tinnitus-related gray matter characteristics in normal-hearing individuals was used, for example, by Mühlau and colleagues (Muhlau et al. 2006) who performed whole-brain VBM analyses and revealed reduced gray matter volume in the subcallosal area of participants affected by tinnitus. Additional regions-of-interest (ROI) analyses only including auditory brain regions also brought to light tinnitus-related increased gray matter density in the right posterior thalamus and the medial geniculate nucleus. However, using a comparable morphometric approach, Landgrebe and colleagues (Landgrebe et al. 2009) were unable to replicate the results, and instead the authors found reduced gray matter volume in individuals with tinnitus in the right inferior colliculus and in the left hippocampus. In a further VBM study, Besteher and colleagues (Besteher et al. 2019) used a ROI-based approach and found decreased gray matter volume as a function of tinnitus in the parahippocampal cortex. Noticeably, with the advent of new computational neuroanatomy tools, some studies harked back to the SBM technique, which bears the advantage of estimating multiple gray matter indices that can be considered as independent traits with different levels of heritability (Rakic 1995, 2000), namely cortical thickness (CT), cortical surface area (CSA) and cortical volume (CV) (Goto et al. 2022). Taking advantage of this approach, Aldhafeeri and colleagues (Aldhafeeri et al. 2012) performed whole-brain and ROI-based SBM analyses, and reported reduced CT in the tinnitus group in a diffuse neural network including the superior, middle and inferior frontal gyri, the anterior cingulate cortex, the cingulate gyrus, the superior, middle and inferior temporal gyri as well as the right primary auditory cortex. Although the results of all these previous anatomical studies are highly heterogeneous, they indicate that tinnitus should be considered as a multidimensional phenomenon related to gray matter peculiarities in sensory, cognitive as well as limbic areas.
The second main branch of research introduced above addressed tinnitus and pure-tone hearing loss as parts of a syndrome, and focused on the comparison between hearing-impaired participants with and without tinnitus (Allan et al. 2016; Benson et al. 2014; Boyen et al. 2013; Husain et al. 2011; Leaver et al. 2012; Schmidt et al. 2018). For example, Boyen and colleagues (Boyen et al. 2013) combined whole-brain and ROI-based VBM analyses, and found increased CV in the left primary auditory cortex of participants with tinnitus compared to the control group. However, this result was only manifested in the ROI analysis, leading to suggest that the whole-brain approach was not sensitive enough or too conservative to detect small between-group differences. In contrast, the whole-brain VBM analysis of Husain and colleagues (Husain et al. 2011) uncovered increased CV in the bilateral superior and medial frontal gyri of participants affected by tinnitus and hearing loss compared to control participants only characterized by hearing loss. In a further structural magnetic resonance imaging (MRI) study which included both VBM and SBM analyses, Leaver and colleagues (Leaver et al. 2012) found that the chronic tinnitus group exhibited reduced CSA in the ventromedial prefrontal cortex, even though this effect was not related to tinnitus distress, depression, anxiety, or noise sensitivity. However, tinnitus distress positively correlated with CT in the anterior insula, while anxiety and depression were negatively associated with CT in the subcallosal area, irrespective of group affiliation. In addition, Allan and co-workers (Allan et al. 2016) applied whole-brain VBM and SBM analyses, and the results indicated decreased CT in the left auditory cortex of tinnitus-affected participants, whereas the VBM analyses did not reveal significant CV differences between the two groups.
Several reasons may account for the somewhat contradictory results emerging from neuroanatomical studies on tinnitus which used different morphometric approaches to identify the crucial gray matter ‘nodes’ responsible for auditory phantom sensations and tinnitus-related distress. A first consideration is that previous work partially included groups that were poorly matched on multiple dimensions, including sample size, age, sex, handedness, education as well as hearing loss. Furthermore, the majority of studies focusing on the joint manifestation of tinnitus and hearing loss only used a pure-tone threshold screener to document the integrity of extra-cortical sources instead of providing a global hearing assessment that takes into account the critical influence of supra-threshold hearing estimates (Fullgrabe et al. 2015; Giroud et al. 2018). Such a drawback is somewhat surprising, than the view that the exclusive assessment of pure-tone thresholds is not enough to describe the full spectrum of hearing capabilities in older age is not at all novel (Dubno et al. 2002; Fullgrabe et al. 2015; Giroud et al. 2018; Humes 2019). Accordingly, proper hearing should rather be considered as an active and integrative process that enables adaptive listening behavior, and that relies on the interplay between the auditory periphery, the central auditory system and brain regions involved in the regulation of higher cognitive functions (Fulton et al. 2015; Giroud et al. 2018). In contrast to pure-tone hearing, supra-threshold hearing more specifically refers to the spectral and temporal resolution properties of the central auditory system that can be measured, for example, by means of frequency selectivity (FS) and temporal compression (TC) tasks (Giroud et al. 2018; Lecluyse et al. 2013). Furthermore, tasks such as speech-in-noise (SiN) recognition imply the additional recruitment of higher cognitive resources, such as attention and working memory, which are necessary to maintain or improve hearing and speech perception in adverse listening conditions (Schmitt et al. 2022; Giroud et al. 2021).
Based on the fact that tinnitus and hearing loss commonly evolve as parts of the same syndrome (Davis and El Rafaie 2000), in the present neuroanatomical study, we used SBM in association with a ROI-based approach, and compared multiple gray matter indices (CT, CSA and CV) between two groups of almost perfectly matched older participants with and without tinnitus, both characterized by pure-tone hearing loss. A crucial aspect of our work is that we took into account the multidimensional facets of hearing, and harmonized the two groups not only in terms of pure-tone hearing but also of supra-threshold hearing parameters that can be measured using TC, FS und SiN tasks (Giroud et al. 2018; Lecluyse et al. 2013; Schmitt et al. 2022). Such a procedure is required to control for confounding effects, because older individuals commonly exhibit heterogenic atrophic profiles (Bethlehem et al. 2022) which are often manifested in poor performance on measures of auditory processing (Fullgrabe 2013; Fullgrabe et al. 2015; Hopkins and Moore 2011; Moore et al. 2014; Pichora-Fuller and Souza 2003), or in speech comprehension difficulties in adverse listening situations despite a pure-tone hearing threshold in the normative range (Fulton et al. 2015). The pre-selected ROIs included brain areas which have previously been proposed to contribute to the emergence and maintenance of tinnitus, tinnitus distress, or to be generally involved in higher order auditory processing (Elmer et al. 2014; Giroud et al. 2018; Kleinjung and Langguth 2020; Meyer et al. 2016; Muhlau et al. 2006; Profant et al. 2020; Rauschecker et al. 2010; Schmidt et al. 2018; Schneider et al. 2009). With this purpose in mind, we specifically focused on the bilateral superior temporal gyrus (STG), Heschl’s gyrus (HG), transverse temporal sulcus (TTS), planum temporale (PT), planum polare (PP), superior temporal sulcus (STS), insula, angular (AG) and supramarginal gyrus (SMG), precuneus, inferior frontal gyrus (IFG, pars opercularis, triangularis and orbitalis), prefrontal cortex (PFC), cingulate cortex (CC), parahippocampal gyrus (PHG), hippocampus and on the amygdala. Furthermore, within the tinnitus group we examined possible associations between gray matter peculiarities, tinnitus duration and distress using assumption-free multiple linear regression analyses that took into account all vertices of the pre-selected ROIs.
Most of the ROIs examined in our work are also reconcilable with current dual-stream models of auditory processing across species (Hickok and Poeppel 2007; Rauschecker and Scott 2009). In fact, results from both humans and monkeys leave little room for doubt about the fundamental role of the ventral stream in decoding spectrally complex sounds and recognizing auditory objects (Rauschecker 2012), whereas the dorsal stream contributes to spatial processes, sensory-motor integration as well as to higher order auditory cognition (Hickok and Poeppel 2007; Rauschecker 2012; Rauschecker and Scott 2009). The neural underpinnings of auditory object recognition and higher order auditory processing also partially overlap across different domains, including speech, music, animal vocalizations and environmental sound recognition (Hickok and Poeppel 2007; Lewis et al. 2004; Rauschecker 2012; Rauschecker and Scott 2009). Hence, it is conceivable to assume that tinnitus may involve hard-wired evolutionary circuits that are additionally shaped by ecological adaptations or external influences, even though this perspective is controversial.
Since tinnitus is closely related to the perception of phantom sounds, we expected to find anatomical between-group differences in primary and associative auditory areas situated in the temporal lobe (Aldhafeeri et al. 2012; Allan et al. 2016; Boyen et al. 2013). Furthermore, based on previous studies indicating a contribution of extra-auditory areas to the maintenance of tinnitus, we predicted that the TIHL group would also be characterized by a differential gray matter architecture in the frontal (Aldhafeeri et al. 2012; Allan et al. 2016; Husain et al. 2011) and parietal (Adjamian et al. 2014; Allan et al. 2016; Meyer et al. 2016) cortex as well as in limbic areas such as the amygdala and hippocampus (Besteher et al. 2019; Landgrebe et al. 2009; Profant et al. 2020; Rauschecker et al. 2010; Tae et al. 2018). However, due to the heterogeneity of previous results, we did not have clear a-priori hypotheses as to whether individuals affected by tinnitus will exhibit increased (Boyen et al. 2013; Husain et al. 2011; Muhlau et al. 2006) or decreased (Aldhafeeri et al. 2012; Allan et al. 2016; Besteher et al. 2019; Landgrebe et al. 2009; Leaver et al. 2012) gray matter parameters in the examined brain areas.
Materials and methods
Participants
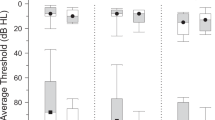
For the present study, we recruited two groups of older participants in the age-range of 63–78 years with (tinnitus and hearing loss, TIHL, N = 33, mean age = 70.51, SD = 3.84) and without (non-tinnitus and hearing loss, NTHL, N = 33, mean age = 69.21, SD = 3.31) tinnitus, but both characterized by mild to moderate pure-tone hearing impairment which was reflected in pure-tone averages (PTA) between 25 and 60 dB hearing loss (Fig. 1). The two groups were matched for sample size, age, sex, handedness, years of education as well as for the degree of pure-tone and supra-threshold hearing loss (i.e., frequency selectivity and temporal compression) as well as for SiN recognition. Furthermore, the two groups demonstrated comparable cognitive capabilities in terms of fluid and crystallized intelligence, processing speed and verbal fluency. The participants of the TIHL group exhibited chronic symptoms for at least 2 years, with a mean tinnitus duration of 19.34 years (SD = 13.21). Furthermore, in the TIHL group, distress was assessed using the tinnitus handicap inventory (THI) (Newman et al. 1996) as well as the tinnitus questionnaire (TQ) (Goebel and Hiller 1994). The demographic, psychometric and audiometric data of the participants are summarized in Table 1.
Pure-tone audiometric profiles. Pure-tone hearing thresholds (dB HL) for the frequencies in the range of 0.5–8 kHz are depicted separately for the tinnitus group with hearing loss (TIHL, green line) and the non-tinnitus group with hearing loss (NTHL, blue line). Each gray line represents an individual participant. dB decibel, HL hearing loss
All participants were native Swiss-German speakers, consistently right-handed (Annett 1970), and demonstrated a score of at least 26 points on the Montreal Cognitive Assessment (MoCA) scale (Nasreddine et al. 2005). None of the participants reported a history of neurological, psychiatric or developmental language disorders, took psychotropic medication, or wore hearing aids. Furthermore, due to possible confounds simultaneous and early bilinguals (Abutalebi and Green 2007; Rodriguez-Fornells et al. 2009) and well as individuals with more than 6 h of music practice per week were excluded (Jancke 2009). The participants were paid for participation, and gave informed written consent in accordance with the procedures of the local ethics committee (Kantonale Ethikkommission Zürich, ethics application number BASEC-NR, 2017-00284) and the declaration of Helsinki.
Cognitive capabilities
To ensure that the two groups exhibited comparable general cognitive capabilities, we tested a specific set of psychometric variables, namely fluid and crystallized intelligence, processing speed and phonemic verbal fluency. Fluid intelligence was examined using the KAI test (‘Kurztest für Allgemeine Basisgrössen der Informationsverarbeitung’) (Lehrl 1992). This procedure consisted of reading aloud meaningless sequences of 20 letters as quickly as possible, and of repeating auditory-presented letters and digits of increasing length (up to nine items). Otherwise, crystallized intelligence was assessed using the MWT-B test (‘Mehrfachwahl-Wortschatz-Intelligenztest’, version B) (Lehrl 1977) which included 37 trials ordered as a function of difficulty level, and the participants had to select the unique word with a meaning from a set of five words that included four pseudowords. Both the KAI and MWT-B tests enable to estimate general intelligence in a short time, and have previously been shown to correlate fairly well (r ~ 0.7) with the global intelligence quotient in healthy adults (Lehrl 1992). Furthermore, processing speed was screened by means of the ZST (‘Zahlen-Symbol-Test’) test which is included in the Wechsler adult intelligence scale (WAIS) (Wechsler 1981). This classical digit-symbol-coding test is a speed-dependent procedure in which simple graphical symbols have to be associated with predetermined numbers in the range of 1–9 as quickly as possible according to an assignment key printed on the test sheet. The test value is calculated based on the number of symbols that are correctly paired with the corresponding numbers in the time range of 120 s. Finally, phonemic verbal fluency was evaluated using the LPS (‘Leistungsprüfsystem’), and consisted of generating as many words as possible beginning with the phonemes /f/, /k/ or /r/ in the time limit of one minute (Horn 1962). Phonemic verbal fluency has been proven to be particularly effective to assess frontal lobe functions (Henry and Crawford 2004). All the four psychometric tests were analyzed and compared between the two groups according to normative T-values.
Hearing and speech-in-noise recognition
To examine participants’ pure-tone and supra-threshold hearing capabilities, we applied established screening procedures that have already been used and described in detail in earlier studies (Giroud et al. 2018; Lecluyse et al. 2013; Wagener et al. 1999). The comprehensive hearing assessment was conducted in a sound booth, and the acoustic stimuli were presented using a Genelec loudspeaker (8030B) positioned at 0° azimuth and at 1.5 m from the subjects’ head. In a first step, we objectified absolute pure-tone thresholds as a correlate of peripheral hearing acuity. According to this procedure, which consisted of a probe-detection paradigm with 250 ms pure-tones presented at 500, 1000, 2000, 4000 and 8000 Hz (Lecluyse et al. 2013), all participants demonstrated pure-tone thresholds of maximal 60 dB hearing loss averaged across the octave frequencies between 500 and 8000 Hz (Fig. 1). In particular, the PTA (averaged over all 5 tested frequencies) was of 35.89 dB hearing loss for the NTHL group (SD = 6.27) and of 35.91 dB for the TIHL group (SD = 6.35).
In a next step, we collected additional hearing estimates using two supra-threshold measurements, namely FS and TC, in association with a forward-masking paradigm (Lecluyse and Meddis 2009; Lecluyse et al. 2013). Forward masking occurs when a probe sound cannot be perceived due to the presence of a preceding masker sound, and such a paradigm is commonly used to identify the masked threshold, which corresponds to the quietest masking tone level still capable of preventing the detection of the probe tone (Lecluyse and Meddis 2009; Lecluyse et al. 2013). In our case, the signal level of the probe tone was fixed, whereas the masker level varied adaptively. The FS task consisted of a masker tone of 108 ms which was followed, after a delay of 10 ms, by a 16 ms probe tone presented at 10 dB above the individual absolute hearing threshold. The participants were asked to determine via button press whether they heard the probe tone or not. Importantly, the intensity level of the masker tone varied according to an adaptive algorithm, and its loudness level either increased or decreased in steps of 2 dB as a function of individuals’ accuracy. This procedure was applied to five probe frequencies (500, 1000, 2000, 4000 and 8000 Hz), and the loudness ratio between the masker and probe tones varied in a predetermined manner (0.7:1, 0.9:1, 1:1, 1.1:1, 1.3:1). Furthermore, to draw attention, each trial was signalized by a cue tone that was presented 500 ms before the masker. For statistical analyses, we calculated the depth between the masker levels at the highest and lowest frequencies, and averaged these values across the five probe frequencies. Such an approach has been shown to provide a valid measure for assessing FS in both normal hearing and hearing-impaired participants (Lecluyse et al. 2013). In addition, TC abilities were measured using the same adaptive forward-masking paradigm with five masker frequencies (500, 1000, 2000, 4000 and 8000 Hz). However, the main difference was that in the TC task the gap between the masker and the probe tones varied adaptively in steps of 10, 30, 50, and 70 ms. The steepness of the psychometric slopes as a function of gap duration were averaged to obtain an objective measure of TC (Lecluyse et al. 2013). Both the FS and TC task were implemented using the MATLAB software.
Finally, as an additional supra-threshold measurement, SiN recognition was assessed using an established task, namely the Oldenburger sentence test (OLSA) (Wagener et al. 1999). This procedure enables to measure the signal-to-noise ratio (SNR) at which a participant is able to correctly reproduce 50% of the words of a sentence embedded in speech-shaped background white noise. Both sentences and noise were initially presented at 65 dB sound pressure level (SPL), and the sentence SPL varied adaptively after each response to assess the SNR. Since the test material consisted of low-constraint sentences, it is unlikely that the participants were able to infer possible candidate words from the context. The auditory stimuli of the SiN task were presented using the MACarena software (https://shorturl.at/ckry7).
MRI data acquisition and surface-based morphometry
High-resolution T1-weighted images were obtained for each participant by means of a 3 Tesla Philips Ingenia scanner (Philips Medical Systems, Best, The Netherlands) equipped with a 12-channel head coil. The three-dimensional anatomical images were acquired using a Turbo-Field-Echo sequence with the following parameters: echo time = 3.7 ms, repetition time = 8.2 ms, field of view = 240 × 240 × 160 mm, acquisition matrix = 240 × 240, 160 slices per volume, isotropic voxel size = 1 × 1 × 1 mm, flip angle (α) = 90°, total scan time = 6 min. No participant was excluded due to inhomogeneous MRI data.
Since the SBM analyses have been performed according to standard procedures and were almost the same as those previously used by our groups (Elmer et al. 2013, 2014; Giroud et al. 2018), some text passages were reiterated from our previous studies. Cortical surface reconstruction was performed with the FreeSurfer image analysis suite (version 7.3.2), which is freely available and documented online (http://freesurfer.net/). The SBM analyses implemented in the FreeSurfer pipeline involved several preprocessing steps, which have already been extensively described in prior publications (Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 1999a, b, 2001, 2002, 2004a, b; Segonne et al. 2004). Briefly, the three-dimensional structural T1-weighted MRI scans were used to construct models of each subject's cortical surface and to measure brain features such as CT, CSA and CV. CT is defined as the minimal distance between gray-white matter borders and the pial surface at each vertex of the tessellated surface (Fischl and Dale 2000). Otherwise, CSA is specified as the mean area of the region at the respective vertex, while CV is simply the arithmetic product of CT and CSA. The FreeSurfer procedure is fully automated and involves segmentation of the cortical white matter (WM) (Dale et al. 1999), tessellation of the gray/white matter junction, inflation of the folded surface tessellation patterns (Fischl et al. 1999a, b), and automatic correction of topological defects in the resulting manifold (Fischl et al. 2001). This surface model was then used as the starting point for a deformable surface algorithm designed to find the gray/white and pial (gray matter/cerebrospinal fluid) surfaces with sub-millimeter precision (Fischl and Dale 2000). Both histological analyses (Rosas et al. 2002) and manual measurements (Kuperberg et al. 2003; Salat et al. 2004) have previously been used to validate the procedure for measuring CT. The method uses both intensity and continuity information from the surfaces in the deformation procedure to be able to interpolate surface information for regions in which the MRI image is ambiguous (Fischl and Dale 2000). For each subject, CT of the cortical ribbon was computed on a uniform grid (comprised by vertices) with 1 mm spacing across both cortical hemispheres, with the thickness being defined by the shortest distance between the gray/white and pial surface models. The CT maps produced are not limited to the voxel resolution of the images and are thus sensitive to detecting sub-millimeter differences between groups (Fischl and Dale 2000). CT measures were mapped onto the inflated surface of each participant’s brain reconstruction, allowing the visualization of the data across the entire cortical surface (i.e., gyri and sulci) without being obscured by the cortical folding pattern. Each subject’s reconstructed brain was then morphed to an average spherical surface representation that optimally aligned sulcal and gyral features across subjects (Fischl et al. 1999a, b). This procedure provides an accurate matching of morphologically homologous cortical locations among participants on the basis of each individual’s anatomy while minimizing metric distortions. This transform was used to map CT and CSA measurements into a common spherical coordinate system. In addition, a parcellation of the cerebral cortex into units based on gyral and sulcal structures was performed (Desikan et al. 2006; Fischl et al. 2004b), and a variety of surface-based data including maps of CT and CSA were created. For all subjects, the data were re-sampled into a common spherical coordinate system (Fischl et al. 1999a, b). The CT and CSA maps were then smoothed on the surface tessellation using an iterative nearest-neighbor averaging procedure (50 iterations were used, which is equivalent to applying a two-dimensional Gaussian smoothing kernel along the cortical surface with a full-width at half-maximum of about 10 mm). These maps were then subjected to group comparisons.
Pre-selection of the ROIs
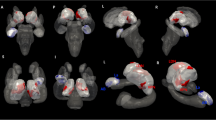
In the present work, we opted for a statistically more lenient ROI approach to maximize statistical power instead of using conservative whole-brain analyses because we did not expect to find relevant group differences beyond the selected regions. The cortex was parcellated into a-priori defined sub-regions according to the aparc.a2009s annotation (Destrieux et al. 2010), and CT, CSA and CV were extracted from 16 bilateral ROIs (Fig. 2), including the CC as well brain areas situated in the frontal (IFG pars opercularis, pars triangularis, pars orbitalis and PFC), the temporal (STG, HG, TTS, PT, PP, STS, insula, PHG) and the parietal (precuneus, AG and SMG) lobes. In addition, we also evaluated the volumes of the hippocampus and the amygdala (Fig. 3), resulting in a total of 18 target ROIs. Thereby, it is noteworthy to mention that instead of evaluating the hippocampus on a gross anatomical scale, we separately examined the volumes of three sub-regions, namely hippocampal head, body and tail (Wisse et al. 2014) using the algorithm by Iglesias et al. (Iglesias et al. 2015) as well as the amygdala (Saygin et al. 2017). The selected brain regions were chosen based on previous work that identified them as relevant for explaining the origin, the maintenance or the emotional distress of tinnitus (Kleinjung and Langguth 2020; Meyer et al. 2016; Muhlau et al. 2006; Profant et al. 2020; Rauschecker et al. 2010; Schmidt et al. 2018; Schneider et al. 2009; Schecklmann et al. 2013).
Sixteen out of 18 regions-of-interest (ROIs) used for the morphometric analyses (without hippocampus and amygdala). First row = lateral view, second row = medial view, third row = anterior view. Left column = right hemisphere, right column = left hemisphere. The number 1–16 represent the 16 regions-of-interest without hippocampus and amygdala
Statistical analyses
Before the main experiment, we used the G*power software to calculate the smallest sample size needed (Faul et al. 2007, 2009). According to this procedure, a mean effect size (partial eta = 0.06) related to the difference in slope between two distinct groups requires a minimum total sample size of N = 54. Since we included two groups of 33 participants each, our sample size was larger than the required minimum. The demographic, psychometric and audiometric data of the participants were evaluated using t tests for independent samples (two-tailed, Table 1) and the IBM SPSS Statistics 26 software package (SPSS, an IBM company, Armonk, New York, USA). The between-group comparison of amygdala and hippocampus (head, body and tail) volumes as well as the multiple linear regressions between these anatomical structures and tinnitus distress (THI)/duration were computed in R (version 4.0.3; R Core Team 2020). In contrast, the between-group comparisons of the 16 remaining cortical ROIs (without hippocampus and amygdala) as well as the corresponding multiple linear regression analyses were conducted in the FreeSurfer environment using the general linear model (GLM) procedure. Importantly, due to the high collinearity between THI and TQ scores (Pearson’s r = 0.851, p < 0.001, two-tailed), possible associations between anatomical parameters and tinnitus distress were only addressed using the THI which has been shown to provide a reliable measure of tinnitus-related emotional states (Newman et al. 1996).
Anatomical analyses: ROI-based group comparisons
For the amygdala and the hippocampal sub-regions, we only examined between-group volume differences by means of separate multiple linear regressions analyses with the factors group and volume, whereas intracranial volume (Z-standardized) was treated as a covariates of no interest. In contrast, the remaining 16 cortical ROIs were compared vertex-wise (CT, CSA and CV) between the two groups. In particular, after having fitted the GLM with the built-in function implemented in FreeSurfer, we ran 10,000 Monte Carlo z-simulations to correct for multiple comparisons at the cluster level (Nichols and Hayasaka 2003) using a vertex-wise/cluster-forming threshold of 0.05 and a cluster-wise p value of 0.05 (Bonferroni-corrected for analyzing both hemispheres). Since the two groups were almost perfectly matched, for all FreeSurfer analyses we did not model any covariates.
Multiple linear regression analyses: brain–tinnitus relationships
To circumvent the problem of circularity and to avoid double dipping (Kriegeskorte et al. 2009), we abstained from computing linear regression analyses by extracting the anatomical clusters that significantly differed between the two groups. Instead, we used an assumption-free procedure, and assessed linear relationships between all vertices included in the 16 ROIs (without hippocampus and amygdala) and THI scores/tinnitus duration. Additional multiple linear regressions were performed to inspect associations between amygdala and hippocampal volumes and the same two behavioral variables of interest. For all regression analyses computed within the TIHL group, sex, age and PTA were modeled as covariates of no interest. Furthermore, the regression analyses focusing on amygdala and hippocampal volumes additionally included intracranial volume as a covariate.
Results
Demographic, psychometric and audiometric data
The statistical analyses of the demographic, psychometric and audiometric data confirmed that the two groups were almost perfectly matched. In fact, we did not reveal significant between-group differences for age, sex, years of education, fluid and crystallized intelligence, processing speed and verbal fluency. In addition, the two groups were also comparable in terms of pure-tone hearing, supra-threshold hearing estimates and SiN performance (Table 1 and Fig. 1).
Anatomical analyses: ROI-based group comparisons
The vertex-wise group comparisons of CT, CSA and CV (16 ROIs without amygdala and hippocampus) revealed increased CV and CSA of the right SMG and the posterior part of the right PT in the TIHL compared to the NTHL group (Fig. 4b, c, Table 2). Furthermore, the TIHL group exhibited a larger CSA in a cluster situated in the middle-anterior part of the left STS (Fig. 4a and Table 2). Otherwise, the multiple linear regression analyses used to compare amygdala and hippocampal (head, body and tail) volumes between the two groups yielded significant results in left-hemispheric structures only. In particular, the TIHL group demonstrated an increased gray matter volume of the amygdala (NTHL vs. TIHL, β = − 74.09, CI = − 141.52/− 6.67, p = 0.032) as well as of the head (NTHL vs. TIHL, β = − 101.30, CI = − 176.05/− 26.54, p = 0.009) and body (NTHL vs. TIHL, β = − 57.76, CI = − 108.67/− 6.85, p = 0.027) of the hippocampus (Fig. 5a, b).
Significant ROI-based results of the vertex-wise comparisons computed in FreeSurfer (without amygdala and hippocampus) between the TIHL (tinnitus and hearing loss) and NTHL (non-tinnitus and hearing loss) groups. The boxplots on the right side depict the single subject data corresponding to the significant clusters, with median and interquartile range. a middle-anterior part of the left superior temporal sulcus (STS), b and c right supramarginal gyrus (SMG) and posterior part of the planum temporale (PT). Color scale = cluster-wise p value (− log10), blue color = TIHL > NTHL, red color = TIHL < NTHL
Volumetric results of the hippocampus (a, head, body and tail) and the amygdala (b), the error bars indicate standard deviation. The volumetric results are shown based on the model prediction values that took into account the influence of intracranial volume (fitted as a covariate of no interest in the multiple linear regressions). NTHL non-tinnitus and hearing loss, TIHL tinnitus and hearing loss. * p < .05, ** p < .01. n.s = not significant
Multiple linear regression analyses: brain–tinnitus relationships
Within the TIHL group, we additionally performed ROI-based (16 ROIs without amygdala and hippocampus) and vertex-wise multiple linear regressions to inspect possible associations between gray matter indices, tinnitus distress (THI) and tinnitus duration (years). According to these analyses, CSA (Fig. 6a and Table 3) as well as CV (Fig. 6b and Table 3) of the left middle-anterior STS and STG were positively related to tinnitus distress. Furthermore, we found a positive association between CSA and THI scores of two clusters situated in the right PFC and posterior STS (Fig. 6c and Table 3). Further multiple linear regressions performed to uncover relationships between gray matter parameters and tinnitus duration revealed positive associations for CSA (Fig. 7a and Table 3) and CV (Fig. 7b and Table 3) in two regions located in the left posterior STS and AG. In contrast, none of the separate multiple linear regression analyses used to inspect volumetric associations between amygdala and hippocampal volumes and tinnitus distress/duration reached significance (all p values > 0.05).
Significant positive relationships within the TIHL (tinnitus and hearing loss) group between gray matter indices and tinnitus distress (THI) in the ROI-based vertex-wise multiple linear regression analyses. a and c cortical surface area, b cortical volume. Color scale = cluster-wise p value (− log10), red = positive association, blue = negative association
ROI-based vertex-wise multiple linear regression analyses, significant positive relationships within the TIHL (tinnitus and hearing loss) group between gray matter indices and tinnitus duration (years). a cortical surface area, b cortical volume. Color scale = cluster-wise p value (− log10), red = positive association, blue = negative association
Discussion
General discussion
The present neuroanatomical study was an attempt to pave the way toward a better understanding of tinnitus as a syndrome characterized by the joint manifestation of auditory phantom sensations and hearing loss. Moreover, we took into account the multifaceted dimensions of hearing and speech processing in adverse listening conditions, and harmonized the two experimental groups not only in terms of hearing thresholds but also of supra-threshold hearing and SiN recognition. The TIHL and NTHL groups were also matched for several critical variables, such as sample size, age, sex, handedness, years of education as well as for general cognitive capabilities. Within this framework, the intertwining of tinnitus and hearing loss was manifested in gray matter peculiarities of perisylvian brain regions, including the right SMG (increased CV and CSA), the right posterior PT (increased CV and CSA) as well as the left middle-anterior part of the STS (increased CSA). In addition, the TIHL group demonstrated larger gray matter volumes of the left amygdala and of the left head and body of the hippocampus. Notably, the examination of brain–tinnitus relationships also brought to light multiple cortical ribbons positively related to tinnitus duration and distress. In particular, CSA of the left middle-anterior STS/STG was predictive of tinnitus distress, and this cluster overlapped with a similar region that turned out to be significant in the group comparison. Tinnitus distress also positively correlated with CV of the left anterior STS/STG, with CSA of the right dorsal PFC as well as with CSA of the right posterior STS. In contrast, tinnitus duration was positively associated with CSA and CV of the right AG and posterior part of the right STS.
Regional morphometric group differences and brain–behavior relationships
Temporal lobe
Using a ROI-based morphometric approach, we were able to replicate a common finding in the tinnitus literature (Boyen et al. 2013; Husain et al. 2011; Landgrebe et al. 2009; Meyer et al. 2016; Schecklmann et al. 2013; Schneider et al. 2009), namely an altered gray matter architecture of the auditory-related cortex, which was manifested in increased CV and CSA of the right posterior part of the PT. Although little is known about the intrinsic meaning of different gray matter parameters, CV can be understood as a gross anatomical dimension that is influenced by the amount and size of neurons, dendrites and glial cells, whereas CSA may reflect cortical folding, gyrification, or even the number and width of cortical microcolumns (Rakic 1995, 2000; van der Meer and Kaufmann 2022; Zilles et al. 1988). However, since the microcolumnar organization of the auditory cortex as well as cortical folding patterns are mostly determined prenatally (Rakic 1995, 2000), it is questionable whether the increased CSA we uncovered in the right PT of the TIHL group can be viewed as a consequence of maladaptive cortical reorganization rather than reflecting anatomical predisposition. Further doubts arise from several studies which examined training-related plasticity across multiple domains, and commonly identified CT or CV, but not mandatorily CSA, as the most malleable gray matter indices (Elmer et al. 2014; Habibi et al. 2018; Langer et al. 2012; Woollett and Maguire 2011). Therefore, our data are not conclusive as to whether the gray matter parameters of the PT reflected either an innate susceptibility to develop tinnitus or rather maladaptive cortical changes induced by environmental or physiological forces.
Interestingly, the increased CV and CSA of the PT were restricted to the right hemisphere, which is consistent with current theories postulating a spectro-temporal division of labor in the associative auditory cortex (Griffiths and Warren 2002; Poeppel 2003; Schonwiesner et al. 2005; Zatorre and Belin 2001). Actually, the PT has been proposed to behave as a computational hub (Griffiths and Warren 2002) that operates in a slightly asymmetric manner, with a preference of the left hemisphere for temporal acoustic analyses and of the right hemisphere for decoding spectral information (Griffiths and Warren 2002; Poeppel 2003; Zatorre and Belin 2001). Given that all participants suffered from pure-tone tinnitus, we may speculate that this distinct auditory phantom sensation was possibly grounded in altered gray matter characteristics of the right PT, which resulted in an over-representation of spectral tinnitus attributes. However, since CV and CSA parameters of this brain region did not correlate with tinnitus distress or symptoms duration, our data strengthen the argument that the gray matter architecture of the right PT constituted a fundamental prerequisite for perceiving tinnitus, whereas its negative connotation and time-dependent amplification were possibly more likely related to auxiliary neural networks. Nevertheless, such a contribution of non-primary auditory areas to vivid auditory sensations in the absence of sound sources in the environment has, for example, also been documented in a variety of auditory imagery studies (Bunzeck et al. 2005; Meyer et al. 2007; Tian et al. 2016). In fact, in contrast to the visual modality (Kosslyn et al. 2001), the imagination of speech, music or environmental sound has convincingly been shown to rely on associative but not primary auditory areas (Bunzeck et al. 2005; Meyer et al. 2007).
As a second morphometric marker, our results highlighted an association between the tinnitus syndrome and CSA of the left middle-anterior part of the STS. The STS is not only one of the longest sulci of the brain (Specht and Wigglesworth 2018), but also a multimodal structure involved in a variety of sensory and cognitive functions, such as phonetic processing, speech and voice perception, audiovisual integration, motion analyses as well as in the discernment of social cues (Belin et al. 2000; Hein and Knight 2008; Specht and Wigglesworth 2018). Interestingly, multimodal processes in the STS have also been shown to follow a gradient along its anterior–posterior axis, with functional activations related to introspection, biological motion and face perception restricted to the posterior portion, and voice, language and general auditory processing confined to the left middle-anterior part (Beauchamp 2015; Binder et al. 2000). Furthermore, lesions in the bilateral STS have been shown to provoke pure auditory agnosia, a condition in which patients are not more able to identify non-speech sounds such as whistling, coughing and crying despite preserved speech comprehension abilities (Clarke et al. 2000; Gutschalk et al. 2015). In addition, the STS has been linked to the categorization of tones (Schulze et al. 2009) and unfamiliar non-phonemic auditory patterns in general (Liebenthal et al. 2010). Such an involvement of the middle-anterior STS in domain-general auditory processing is not surprising, especially in light of previous work showing that the STS fulfills cyto- and receptor-architectonic properties common to sensory cortical fields (Morosan et al. 2005).
Importantly, the assumption-free multiple linear regression analyses also uncovered a positive relationship between CSA of the left middle-anterior STS/STG and tinnitus distress, and this cluster overlapped with a similar gray matter node that significantly differed between the two groups. Accordingly, our data emphasize that the gray matter infrastructure underlying chronic tinnitus can be part of the same matrix which is also involved in aversive or distressed reactions to tinnitus. Nevertheless, the precise functional role of the left middle-anterior STS in tinnitus distress remains purely elusive. One possible interpretation is that the anatomical peculiarities of this brain region were associated with a disturbance of the functional balance in the ventral processing stream mainly involved in auditory object recognition (Beauchamp et al. 2004; Werner and Noppeney 2010). In this vein, we may speculate whether such gray matter anomalies possibly reinforced the binding of tinnitus characteristics, or the maintenance of tinnitus in memory due to the misrepresentation of tone-specific auditory objects characterized by a distinctive sound quality and a defined spatial location (Griffiths and Warren 2004; Snyder and Alain 2007). This view would also be compatible with the idea that the between-group differences we revealed in the left middle-anterior part of the STS possibly reflected a change in tonotopic organization. In fact, even though a faithful topographic representation of frequencies is commonly attributed to the cochlea, to the subcortical nuclei, and to a lesser extent to the primary auditory cortex, there is evidence showing that tonotopic maps also co-exist in the STS (Cansino et al. 1994; Castro and Kandler 2010). The two perspectives sketched above would also be compatible with the additional results of the multiple linear regression analyses indicating that tinnitus distress correlated with CV in the left anterior STS/STG as well as with CSA in the right posterior STS.
Parietal lobe
The third main finding of our study was an increased CV and CSA of the right SMG in the TIHL compared to the NTHL group. This right-sided brain region, together with the AG, is part of the so-called temporo-parietal junction (TPJ) (Krall et al. 2015), plays a fundamental role in the orientation of attention toward new stimuli, and has been associated with contralateral neglect in both the visual and auditory modalities (Gutschalk and Dykstra 2015; Horiguchi et al. 2016; Karnath and Rorden 2012). The right TPJ has also been shown to be crucially involved in the processing of emotional information, and to contribute to emotional experiences (Dzafic et al. 2019; Grecucci et al. 2013; Zaitchik et al. 2010). According to the model proposed by Heller (1993), the right TPJ interacts with the prefrontal cortex to regulate emotional states through the modulation of valence and arousal. In particular, the theory postulates an asymmetry of emotional valence in the prefrontal cortex, with an involvement of the left hemisphere in positive and of the right hemisphere in negative emotion regulation. Furthermore, the right TPJ plays an important role in the guidance of arousal, which refers to a psychological state of alertness or excitation, for example, in association with stress, fear or anger (Messina et al. 2017). Based on this framework, we speculate that the increased CV and CSA of the right SMG in the TIHL group may have resulted in increased levels of arousal through the activation of the autonomic nervous system, while at the same time reducing the ability to ignore or filter out tinnitus-related information (McKenna et al. 2014). Although we did not reveal a positive relationship between anatomical indices of the right SMG and tinnitus characteristics, tinnitus duration was at least positively related to CSA and CV of the right AG. Furthermore, tinnitus distress positively correlated with CSA of the right dorsal PFC, possibly indicating that the right TPJ and the PFC are crucial components of a tinnitus-related valence-arousal network.
Amygdala and hippocampus
As a fourth major finding, the multiple linear regression models used to assess between-group differences in the amygdala and hippocampus only yielded left-hemispheric outcomes. In particular, the TIHL group exhibited an increased gray matter volume of the amygdala as well as of the head and body of the hippocampus. While anatomical changes in these two brain structures have already been documented in previous studies (Kraus and Canlon 2012; Profant et al. 2020; Zhang et al. 2019), the novel aspect of our work is that we provided a more comprehensive understanding of the hippocampal sub-regions related to tinnitus. Despite the fact that the hippocampus is classically considered the site of episodic and spatial memory formation (Scoville and Milner 1957; Squire and Zolamorgan 1991), recent perspectives also emphasized a fundamental role of this brain region in auditory cognition (Billig et al. 2022). Indeed, hippocampal responses to sounds have been observed in different domains, including passive exposure (Brown and Buchwald 1973), active listening (Kumar et al. 2014), and associative auditory learning (Derner et al. 2020). Furthermore, the hippocampus has been shown to support the binding of auditory features with disparate elements across both space and time (Olsen et al. 2012), and to be involved in speech (Urgolites et al. 2022) and music processing (Muller et al. 2013; Urgolites et al. 2022). Such a hippocampal contribution to multiple auditory domains is not entirely surprising, especially given its mutual connections with the auditory cortex (Billig et al. 2022). In this regard, both animal and human studies provided evidence for numerous functional and anatomical pathways from the auditory brainstem and the medial geniculate body to the hippocampus, and from there to auditory cortical core and belt areas (Jang and Choi 2022). Conversely, many circuits are also available for auditory cortex activity to reach the hippocampus, resulting in multiple reciprocal interactions (Billig et al. 2022).
Interestingly, even if the organization of the hippocampus along its longitudinal axis is somewhat controversial (Genon et al. 2021), several neuroimaging findings postulated the existence of functional sub-regions (Daugherty et al. 2016) which are legitimated by distinct functional and structural connectivity profiles (Adnan et al. 2016). Principally, the anterior part of the hippocampus, including head and body, has substantial connections with the amygdala, lateral temporal regions, the STG, the temporal pole, the insula as well as with the anterior CC and ventromedial PFC via the fornix-mammillary body-thalamic system and the uncinate fasciculus (Kahn et al. 2008; Wang et al. 2016). In contrast, the posterior portion of the hippocampus, including the tail, is mainly linked to frontal and parietal cortical regions (Poppenk et al. 2013).
Not only the hippocampus but also the amygdala is involved in auditory cognition, and is particularly susceptible to sounds with a valence, such as vocalizations, crying or music (Kraus and Canlon 2012). Furthermore, the amygdala is sensitive to auditory fear conditioning (Holland and Gallagher 1999; Rasia-Filho et al. 2000; Savage and Ramos 2009) and relevance detection (Sander et al. 2003; Zald 2003), and has been shown to be implicated in the regulation of attention (Gallagher and Holland 1994; Pessoa 2011), arousal (Davis and Whalen 2001; Murray and Wise 2010) as well as to modulate neuroplastic processes in the auditory cortex (Kraus and Canlon 2012). Importantly, acoustic stimuli characterized by a negative connotation have also been shown to induce the release of stress hormones via the hypothalamic–pituitary–adrenal axis (Kraus and Canlon 2012), and long-term exposure to glucocorticoids is known to promote synaptic loss, neural shrinkage and general gray matter atrophy (Nichols et al. 2001; Sapolsky et al. 1990).
Based on literature reviewed above, it seems obvious that the amygdala–hippocampal system deserves serious consideration for a more complete understanding of the neural circuits involved in the generation and maintenance of tinnitus beyond the auditory cortex (Kraus and Canlon 2012; Zhang et al. 2019). In line with this, the automatic FreeSurfer-based tripartition of the bilateral hippocampus only resulted in between-group volume differences in the left hemisphere, and this distinctive anatomical pattern was restricted to the anterior compartments consisting of head and body parts. In unison with the hippocampal results, amygdala volume differences between the two groups were also exclusively found in the left hemisphere. While it was beyond the scope of this study to determine the cause of distinct results in the two hemispheres, the common volumetric between-group differences we found in the anterior parts of the hippocampus and in the amygdala are possibly due to close connections between these two medial temporal lobe structures (Kahn et al. 2008; Wang et al. 2016). Hence, it is possible that anatomically predetermined connectivity patterns may have contributed to mutual volumetric changes in both brain structures, which resulted in a joint dysfunctional unit. It is also conceivable that tinnitus-related distress activated the hypothalamic–pituitary–adrenal axis and stimulated glucocorticoid release from the adrenal gland, which bound to the respective receptors in the limbic system, including the hippocampus and the amygdala (Roozendaal et al. 1999; Wang et al. 2014). However, although these two tinnitus-related brain regions are important targets for glucocorticoids and stress, high cortisol levels are commonly associated with decreased and not increased gray matter volumes in the hippocampus and the amygdala, especially due to a degradation of dendrites and synapses (Tata and Anderson 2010). Furthermore, the volumetric indices of the amygdala and of the head and body of the hippocampus were not predictive of tinnitus-related distress (THI), which makes it difficult to believe that the anatomical modifications were determined by glucocorticoids.
Although the increased amygdala and hippocampus volumes we revealed in the TIHL group are in line with a previous anatomical study of Profant and colleagues (Profant et al. 2020), the interpretation of these results is not straightforward. Nevertheless, there are several other factors that may have contributed to these findings. A first consideration is that the increased volumes of the amygdala and hippocampus were possibly induced by a tinnitus-related overstimulation of auditory cortical fields due to maladaptive reorganization of tonotopic maps (Kraus and Canlon 2012; Okamoto et al. 2010). Since auditory stimulation is essential for the development of amygdala and hippocampal functions (Billig et al. 2022; Zhang et al. 2022), it might have been the case that auditory overstimulation promoted an upregulation of receptors due to changes in neurotransmission, or even a spreading of blood vessels, all this resulting in increased structural volumes (Wasserthal et al. 2014; Zhang et al. 2022). Despite this, it is also possible that the common structural changes of the amygdala and the hippocampus were related to the sustainability of the tinnitus symptoms which were possibly mediated by the reciprocal dependence between the two brain structures (Billig et al. 2022). In this light, it might be that amygdala and hippocampus merged to a dysfunctional circuitry that triggered the maintenance of tinnitus through an amygdala-dependent orientation of attention (Gallagher and Holland 1994; Rasia-Filho et al. 2000), arousal (Davis and Whalen 2001; Murray and Wise 2010) or emotional appraisal toward the phantom sensations which benefited the long-term memory storage of tinnitus-related auditory information in the hippocampus (De Ridder et al. 2006; Naber et al. 2000).
Finally, it is noteworthy to mention that the discrepancy that exists in the literature regarding increased or decreased gray matter parameters and tinnitus results in general might be related to the high inter-individual variability of symptom manifestations (Cederroth et al. 2019) as well as to personal experiences in dealing with the syndrome. Therefore, future studies should mandatorily take into account the individual facets of tinnitus by combining functional and structural imaging protocols with comprehensive psychometric measurements. In this context, a major breakthrough in the field of pure-tone tinnitus research could be achieved, for example, using a multistep approach consisting of determining the individual tinnitus frequencies, of identifying the individual functional matrices responsive to pure-tone stimulation in the same frequency range, and of extracting and comparing gray matter parameters of the brain regions showing the highest degree of functional overlap. We are confident that such a procedure will open new avenues that are expected to enrich the understanding of tinnitus as a syndrome.
Conclusions
Previous anatomical studies mostly examined tinnitus in isolation without considering auditory phantom sensations as part of a syndrome in comorbidity with pure-tone hearing loss. Furthermore, none of the previous studies took into account the multidimensional facets of hearing, and matched the groups for pure-tone hearing loss as well as for supra-threshold hearing estimates and SiN performance. Using a ROI approach, we identified the PT, the STS and the TPJ as fundamental hallmarks of the tinnitus syndrome matrix, which operates in interaction with other auxiliary brain regions. This specific pattern of outcomes leads us to believe that the PT plays a critical role in evoking the sensation of pure-tone ghosting, the TPJ in reinforcing the arousal drive for tinnitus-related features, and the STS in binding together all the multifaceted dimensions. Furthermore, the amygdala–hippocampus complex and the PFC might contribute to the regulation of valence and arousal levels. Finally, it is noteworthy to emphasize that due to the close spatial correspondence between-group-level results and brain–behavior relationships in the middle-anterior portion of the left STS, this multimodal territory meets the requirements of being a core area of the tinnitus syndrome matrix.
Data and code availability
We reported all measures and conditions without data exclusions. All codes and data are available from MM and RS. Due to ethical considerations, the MRI data cannot be made openly available. However, upon reasonable request, the MRI and behavioral data will be shared without any restrictions.
References
Abutalebi J, Green D (2007) Bilingual language production: the neurocognition of language representation and control. J Neurolinguist 20(3):242–275. https://doi.org/10.1016/j.jneuroling.2006.10.003
Adjamian P, Hall DA, Palmer AR, Allan TW, Langers DRM (2014) Neuroanatomical abnormalities in chronic tinnitus in the human brain. Neurosci Biobehav R 45:119–133. https://doi.org/10.1016/j.neubiorev.2014.05.013
Adnan A, Barnett A, Moayedi M, McCormick C, Cohn M, McAndrews MP (2016) Distinct hippocampal functional networks revealed by tractography-based parcellation. Brain Struct Funct 221(6):2999–3012. https://doi.org/10.1007/s00429-015-1084-x
Aldhafeeri FM, Mackenzie I, Kay T, Alghamdi J, Sluming V (2012) Neuroanatomical correlates of tinnitus revealed by cortical thickness analysis and diffusion tensor imaging. Neuroradiology 54(8):883–892. https://doi.org/10.1007/s00234-012-1044-6
Allan TW, Besle J, Langers DRM, Davies J, Hall DA, Palmer AR, Adjamian P (2016) Neuroanatomical alterations in tinnitus assessed with magnetic resonance imaging. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2016.00221
Annett M (1970) A classification of hand preference by association analysis. Br J Psychol 61(3):303–310. https://doi.org/10.1111/j.2044-8295.1970.tb01248.x
Araneda R, Renier L, Dricot L, Decat M, Ebner-Karestinos D, Deggouj N, De Volder AG (2018) A key role of the prefrontal cortex in the maintenance of chronic tinnitus: An fMRI study using a Stroop task. Neuroimage-Clin 17:325–334. https://doi.org/10.1016/j.nicl.2017.10.029
Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M (1996) Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec 58(4):195–199. https://doi.org/10.1159/000276835
Axelsson A, Ringdahl A (1989) Tinnitus–a study of its prevalence and characteristics. Br J Audiol 23(1):53–62. https://doi.org/10.3109/03005368909077819
Baguley D, McFerran D, Hall D (2013) Tinnitus. Lancet 382(9904):1600–1607. https://doi.org/10.1016/S0140-6736(13)60142-7
Batista-Garcia-Ramo K, Fernandez-Verdecia CI (2018) What we know about the brain structure-function relationship. Behav Sci Basel. https://doi.org/10.3390/bs8040039
Beauchamp MS (2015) The social mysteries of the superior temporal sulcus. Trends Cogn Sci 19(9):489–490. https://doi.org/10.1016/j.tics.2015.07.002
Beauchamp MS, Lee KE, Argall BD, Martin A (2004) Integration of auditory and visual information about objects in superior temporal sulcus. Neuron 41(5):809–823. https://doi.org/10.1016/S0896-6273(04)00070-4
Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B (2000) Voice-selective areas in human auditory cortex. Nature 403(6767):309–312. https://doi.org/10.1038/35002078
Benson RR, Gattu R, Cacace AT (2014) Left hemisphere fractional anisotropy increase in noise-induced tinnitus: a diffusion tensor imaging (DTI) study of white matter tracts in the brain. Hearing Res 309:8–16. https://doi.org/10.1016/j.heares.2013.10.005
Besteher B, Gaser C, Ivansic D, Guntinas-Lichius O, Dobel C, Nenadic I (2019) Chronic tinnitus and the limbic system: reappraising brain structural effects of distress and affective symptoms. Neuroimage Clin. https://doi.org/10.1016/j.nicl.2019.101976
Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, Adler S, Alexopoulos GS, Anagnostou E, Areces-Gonzalez A, Astle DE, Auyeung B, Ayub M, Bae J, Ball G, Baron-Cohen S, Beare R, Bedford SA, Benegal V, Beyer F, Blangero J, Cabez MB, Boardman JP, Borzage M, Bosch-Bayard JF, Bourke N, Calhoun VD, Chakravarty MM, Chen C, Chertavian C, Chetelat G, Chong YS, Cole JH, Corvin A, Costantino M, Courchesne E, Crivello F, Cropley VL, Crosbie J, Crossley N, Delarue M, Delorme R, Desrivieres S, Devenyi GA, Di Biase MA, Dolan R, Donald KA, Donohoe G, Dunlop K, Edwards AD, Elison JT, Ellis CT, Elman JA, Eyler L, Fair DA, Feczko E, Fletcher PC, Fonagy P, Franz CE, Galan-Garcia L, Gholipour A, Giedd J, Gilmore JH, Glahn DC, Goodyer IM, Grant PE, Groenewold NA, Gunning FM, Gur RE, Gur RC, Hammill CF, Hansson O, Hedden T, Heinz A, Henson RN, Heuer K, Hoare J, Holla B, Holmes AJ, Holt R, Huang H, Im K, Ipser J, Jack CR, Jackowski AP, Jia T, Johnson KA, Jones PB, Jones DT, Kahn RS, Karlsson H, Karlsson L, Kawashima R, Kelley EA, Kern S, Kim KW, Kitzbichler MG, Kremen WS, Lalonde F, Landeau B, Lee S, Lerch J, Lewis JD, Li J, Liao W, Liston C, Lombardo MV, Lv J, Lynch C, Mallard TT, Marcelis M, Markello RD, Mathias SR, Mazoyer B, McGuire P, Meaney MJ, Mechelli A, Medic N, Misic B, Morgan SE, Mothersill D, Nigg J, Ong MQW, Ortinau C, Ossenkoppele R, Ouyang M, Palaniyappan L, Paly L, Pan PM, Pantelis C, Park MM, Paus T, Pausova Z, Paz-Linares D, Binette AP, Pierce K, Qian X, Qiu J, Qiu A, Raznahan A, Rittman T, Rodrigue A, Rollins CK, Romero-Garcia R, Ronan L, Rosenberg MD, Rowitch DH, Salum GA, Satterthwaite TD, Schaare HL, Schachar RJ, Schultz AP, Schumann G, Scholl M, Sharp D, Shinohara RT, Skoog I, Smyser CD, Sperling RA, Stein DJ, Stolicyn A, Suckling J, Sullivan G, Taki Y, Thyreau B, Toro R, Traut N, Tsvetanov KA, Turk-Browne NB, Tuulari JJ, Tzourio C, Vachon-Presseau E, Valdes-Sosa MJ, Valdes-Sosa PA, Valk SL, van Amelsvoort T, Vandekar SN, Vasung L, Victoria LW, Villeneuve S, Villringer A, Vertes PE, Wagstyl K, Wang YS, Warfield SK, Warrier V, Westman E, Westwater ML, Whalley HC, Witte AV, Yang N, Yeo B, Yun H, Zalesky A, Zar HJ, Zettergren A, Zhou JH, Ziauddeen H, Zugman A, Zuo XN, Bullmore ET, Alexander-Bloch AF, 3R-BRAIN, AIBL, Initi AsDN, Borders AsDR, Team C, Cam-CAN, CCNP, COBRE, cVEDA, Grp EDBAW, Projec DHC, FinnBrain, Study HAB, IMAGEN, KNE96, Aging MCS, NSPN, POND, Grp P-AR, VETSA (2022) Brain charts for the human lifespan. Nature 604(7906):525–533. https://doi.org/10.1038/s41586-022-04554-y
Billig AJ, Lad M, Sedley W, Griffiths TD (2022) The hearing hippocampus. Prog Neurobiol. https://doi.org/10.1016/j.pneurobio.2022.102326
Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET (2000) Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10(5):512–528. https://doi.org/10.1093/cercor/10.5.512
Boyen K, Langers DR, de Kleine E, van Dijk P (2013) Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear Res 295:67–78. https://doi.org/10.1016/j.heares.2012.02.010
Brown KA, Buchwald JS (1973) Acoustic responses and plasticity of limbic units in cats. Exp Neurol 40(3):608–631. https://doi.org/10.1016/0014-4886(73)90099-X
Bunzeck N, Wuestenberg T, Lutz K, Heinze HJ, Jancke L (2005) Scanning silence: mental imagery of complex sounds. Neuroimage 26(4):1119–1127. https://doi.org/10.1016/j.neuroimage.2005.03.013
Cansino S, Williamson SJ, Karron D (1994) Tonotopic organization of human auditory association cortex. Brain Res 663(1):38–50. https://doi.org/10.1016/0006-8993(94)90460-X
Carpenter-Thompson JR, Schmidt SA, Husain FT (2015) Neural plasticity of mild tinnitus: an fMRI investigation comparing those recently diagnosed with tinnitus to those that had tinnitus for a long period of time. Neural Plast. https://doi.org/10.1155/2015/161478
Castro JB, Kandler K (2010) Changing tune in auditory cortex. Nat Neurosci 13(3):271–273. https://doi.org/10.1038/nn0310-271
Cederroth CR, Gallus S, Hall DA, Kleinjung T, Langguth B, Maruotti A, Meyer M, Norena A, Probst T, Pryss R, Searchfield G, Shekhawat G, Spiliopoulou M, Vanneste S, Schlee W (2019) Editorial: towards an understanding of tinnitus heterogeneity. Front Aging Neurosci 11:53. https://doi.org/10.3389/fnagi.2019.00053
Cheng SR, Xu GX, Zhou J, Qu YZ, Li ZJ, He ZX, Yin T, Ma PH, Sun RR, Liang FR (2020) A multimodal meta-analysis of structural and functional changes in the brain of tinnitus. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2020.00028
Clarke S, Bellmann A, Meuli RA, Assal G, Steck AJ (2000) Auditory agnosia and auditory spatial deficits following left hemispheric lesions: evidence for distinct processing pathways. Neuropsychologia 38(6):797–807. https://doi.org/10.1016/S0028-3932(99)00141-4
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis – I. Segmentation and surface reconstruction. Neuroimage 9(2):179–194. https://doi.org/10.1006/nimg.1998.0395
Daugherty AM, Bender AR, Raz N, Ofen N (2016) Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus 26(2):220–228. https://doi.org/10.1002/hipo.22517
Davis A, El Rafaie A (2000) Epidemiology of tinnitus. Tinnitus Handbook. RS Tyler Singular Thomson Learning, San Diego, pp 1–23
Davis M, Whalen PJ (2001) The amygdala: vigilance and emotion. Mol Psychiatr 6(1):13–34. https://doi.org/10.1038/sj.mp.4000812
De Ridder D, Fransen H, Francois O, Sunaert S, Kovacs S, Van De Heyning P (2006) Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Oto-Laryngol 126:50–53. https://doi.org/10.1080/03655230600895580
De Ridder D, Elgoyhen AB, Romo R, Langguth B (2011) Phantom percepts: Tinnitus and pain as persisting aversive memory networks. P Natl Acad Sci USA 108(20):8075–8080. https://doi.org/10.1073/pnas.1018466108
Derner M, Dehnen G, Chaieb L, Reber TP, Borger V, Surges R, Staresina BP, Mormann F, Fell J (2020) Patterns of single-neuron activity during associative recognition memory in the human medial temporal lobe. Neuroimage. https://doi.org/10.1016/j.neuroimage.2020.117214
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Destrieux C, Fischl B, Dale A, Halgren E (2010) Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53(1):1–15. https://doi.org/10.1016/j.neuroimage.2010.06.010
Dubno JR, Horwitz AR, Ahlstrom JB (2002) Benefit of modulated maskers for speech recognition by younger and older adults with normal hearing. J Acoust Soc Am 111(6):2897–2907. https://doi.org/10.1121/1.1480421
Dzafic I, Oestreich L, Martin AK, Mowry B, Burianova H (2019) Stria terminalis, amygdala, and temporoparietal junction networks facilitate efficient emotion processing under expectations. Hum Brain Mapp 40(18):5382–5396. https://doi.org/10.1002/hbm.24779
Eggermont JJ (2007) Pathophysiology of tinnitus. Prog Brain Res 166:19–36. https://doi.org/10.1016/S0079-6123(07)66002-6
Eggermont JJ (2008) The role of sound in adult and developmental auditory cortical plasticity. Ear Hear 29(6):819–829. https://doi.org/10.1097/AUD.0b013e3181853030
Eichhammer P, Langguth B, Marienhagen J, Kleinjung T, Hajak G (2003) Neuronavigated repetitive transcranial magnetic stimulation in patients with tinnitus: a short case series. Biol Psychiatry 54(8):862–865. https://doi.org/10.1016/s0006-3223(02)01896-6
Elmer S, Hanggi J, Meyer M, Jancke L (2013) Increased cortical surface area of the left planum temporale in musicians facilitates the categorization of phonetic and temporal speech sounds. Cortex 49(10):2812–2821. https://doi.org/10.1016/j.cortex.2013.03.007
Elmer S, Hanggi J, Jancke L (2014) Processing demands upon cognitive, linguistic, and articulatory functions promote grey matter plasticity in the adult multilingual brain: Insights from simultaneous interpreters. Cortex 54:179–189. https://doi.org/10.1016/j.cortex.2014.02.014
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191. https://doi.org/10.3758/Bf03193146
Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41(4):1149–1160. https://doi.org/10.3758/BRM.41.4.1149
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. P Natl Acad Sci USA 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Fischl B, Sereno MI, Dale AM (1999a) Cortical surface-based analysis - II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9(2):195–207. https://doi.org/10.1006/nimg.1998.0396
Fischl B, Sereno MI, Tootell RBH, Dale AM (1999b) High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8(4):272–284. https://doi.org/10.1002/(Sici)1097-0193(1999)8:4%3c272::Aid-Hbm10%3e3.0.Co;2-4
Fischl B, Liu A, Dale AM (2001) Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. Ieee T Med Imaging 20(1):70–80. https://doi.org/10.1109/42.906426
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355. https://doi.org/10.1016/S0896-6273(02)00569-X
Fischl B, Salat DH, van der Kouwe AJW, Makris N, Segonne F, Quinn BT, Dale AM (2004a) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23:S69–S84. https://doi.org/10.1016/j.neuroimage.2004.07.016
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004b) Automatically parcellating the human cerebral cortex. Cereb Cortex 14(1):11–22. https://doi.org/10.1093/cercor/bhg087
Fullgrabe C (2013) Age-dependent changes in temporal-fine-structure processing in the absence of peripheral hearing loss. Am J Audiol 22(2):313–315. https://doi.org/10.1044/1059-0889(2013/12-0070)
Fullgrabe C, Moore BCJ, Stone MA (2015) Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2014.00347
Fulton SE, Lister JJ, Bush AL, Edwards JD, Andel R (2015) Mechanisms of the hearing-cognition relationship. Semin Hear 36(3):140–149. https://doi.org/10.1055/s-0035-1555117
Gallagher M, Holland PC (1994) The amygdala complex - multiple roles in associative learning and attention. P Natl Acad Sci USA 91(25):11771–11776. https://doi.org/10.1073/pnas.91.25.11771
Genon S, Bernhardt BC, La Joie R, Amunts K, Eickhoff SB (2021) Review the many dimensions of human hippocampal organization and (dys)function. Trends Neurosci 44(12):977–989. https://doi.org/10.1016/j.tins.2021.10.003
Giraud AL, Chery-Croze S, Fischer G, Fischer C, Vighetto A, Gregoire MC, Lavenne F, Collet L (1999) A selective imaging of tinnitus. NeuroReport 10(1):1–5. https://doi.org/10.1097/00001756-199901180-00001
Giroud N, Hirsiger S, Muri R, Kegel A, Dillier N, Meyer M (2018) Neuroanatomical and resting state EEG power correlates of central hearing loss in older adults. Brain Struct Funct 223(1):145–163. https://doi.org/10.1007/s00429-017-1477-0
Giroud N, Keller M, Meyer M (2021) Interacting effects of frontal lobe neuroanatomy and working memory capacity to older listeners’ speech recognition in noise. Neuropsychologia. https://doi.org/10.1016/j.neuropsychologia.2021.107892
Goebel G, Hiller W (1994) The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO 42(3):166–172
Golm D, Schmidt-Samoa C, Dechent P, Kroner-Herwig B (2013) Neural correlates of tinnitus related distress: an fMRI-study. Hearing Res 295:87–99. https://doi.org/10.1016/j.heares.2012.03.003
Goto M, Abe O, Hagiwara A, Fujita S, Kamagata K, Hori M, Aoki S, Osada T, Konishi S, Masutani Y, Sakamoto H, Sakano Y, Kyogoku S, Daida H (2022) Advantages of using both voxel- and surface-based morphometry in cortical morphology analysis: a review of various applications. Magn Reson Med Sci 21(1):41–57. https://doi.org/10.2463/mrms.rev.2021-0096
Grecucci A, Giorgetta C, Bonini N, Sanfey AG (2013) Reappraising social emotions: the role of inferior frontal gyrus, temporo- parietal junction and insula in interpersonal emotion regulation. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2013.00523
Griffiths TD, Warren JD (2002) The planum temporale as a computational hub. Trends Neurosci 25(7):348–353. https://doi.org/10.1016/S0166-2236(02)02191-4
Griffiths TD, Warren JD (2004) What is an auditory object? Nat Rev Neurosci 5(11):887–892. https://doi.org/10.1038/nrn1538
Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR (2010) Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104(6):3361–3370. https://doi.org/10.1152/jn.00226.2010
Gutschalk A, Dykstra A (2015) Auditory neglect and related disorders. Handb Clin Neurol 129:557–571. https://doi.org/10.1016/B978-0-444-62630-1.00031-7
Gutschalk A, Uppenkamp S, Riedel B, Bartsch A, Brandt T, Vogt-Schaden M (2015) Pure word deafness with auditory object agnosia after bilateral lesion of the superior temporal sulcus. Cortex 73:24–35. https://doi.org/10.1016/j.cortex.2015.08.001
Habibi A, Damasio A, Ilari B, Veiga R, Joshi AA, Leahy RM, Haldar JP, Varadarajan D, Bhushan C, Damasio H (2018) Childhood music training induces change in micro and macroscopic brain structure: results from a longitudinal study. Cereb Cortex 28(12):4336–4347. https://doi.org/10.1093/cercor/bhx286
Hein G, Knight RT (2008) Superior temporal sulcus-it’s my area: or is it? J Cognitive Neurosci 20(12):2125–2136. https://doi.org/10.1162/jocn.2008.20148
Heller W (1993) Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology 7:476
Henry JD, Crawford JR (2004) A meta-analytic review of verbal fluency performance in patients with traumatic brain injury. Neuropsychology 18(4):621–628. https://doi.org/10.1037/0894-4105.18.4.621
Hickok G, Poeppel D (2007) Opinion - the cortical organization of speech processing. Nat Rev Neurosci 8(5):393–402. https://doi.org/10.1038/nrn2113
Holland PC, Gallagher M (1999) Amygdala circuitry in attentional and representational processes. Trends Cogn Sci 3(2):65–73. https://doi.org/10.1016/S1364-6613(98)01271-6
Hopkins K, Moore BCJ (2011) The effects of age and cochlear hearing loss on temporal fine structure sensitivity, frequency selectivity, and speech reception in noise. J Acoust Soc Am 130(1):334–349. https://doi.org/10.1121/1.3585848
Horiguchi H, Wandell BA, Winawer J (2016) A predominantly visual subdivision of the right temporo-parietal junction (vTPJ). Cereb Cortex 26(2):639–646. https://doi.org/10.1093/cercor/bhu226
Horn W (1962) Leistungsprüfsystem, L-P-S: Handanweisung für die Durchführung, Auswertung und Interpretation [A performance testing system: Manual for administration, scoring, and interpretation]. Verlag Hogrefe
House J, Brackmann D (1981) Tinnitus: surgical treatment. Tinnitus (Ciba Foundation Symposium 85). Ciba Foundation Symposium 85
Humes LE (2019) The World Health Organization’s hearing-impairment grading system: an evaluation for unaided communication in age-related hearing loss. Int J Audiol 58(1):12–20. https://doi.org/10.1080/14992027.2018.1518598
Husain FT, Medina RE, Davis CW, Szymko-Bennett Y, Simonyan K, Pajor NM, Horwitz B (2011) Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res 1369:74–88. https://doi.org/10.1016/j.brainres.2010.10.095
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, Roy N, Frosch MP, McKee AC, Wald LL, Fischl B, Van Leemput K, Neuroimaging AD (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115:117–137. https://doi.org/10.1016/j.neuroimage.2015.04.042
Jancke L (2009) The plastic human brain. Restor Neurol Neuros 27(5):521–538. https://doi.org/10.3233/Rnn-2009-0519
Jang SH, Choi EB (2022) Evaluation of structural neural connectivity between the primary auditory cortex and cognition-related brain areas using diffusion tensor tractography in 43 normal adults. Med Sci Monitor. https://doi.org/10.12659/MSM.936131
Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL (2008) Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol 100(1):129–139. https://doi.org/10.1152/jn.00077.2008
Karnath HO, Rorden C (2012) The anatomy of spatial neglect. Neuropsychologia 50(6):1010–1017. https://doi.org/10.1016/j.neuropsychologia.2011.06.027
Kleinjung T, Langguth B (2020) Avenue for future tinnitus treatments. Otolaryng Clin N Am 53(4):667–683. https://doi.org/10.1016/j.otc.2020.03.013
Kosslyn SM, Ganis G, Thompson WL (2001) Neural foundations of imagery. Nat Rev Neurosci 2(9):635–642. https://doi.org/10.1038/35090055
Krall SC, Rottschy C, Oberwelland E, Bzdok D, Fox PT, Eickhoff SB, Fink GR, Konrad K (2015) The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct Funct 220(2):587–604. https://doi.org/10.1007/s00429-014-0803-z
Kraus KS, Canlon B (2012) Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288(1–2):34–46. https://doi.org/10.1016/j.heares.2012.02.009
Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI (2009) Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 12(5):535–540. https://doi.org/10.1038/nn.2303
Kumar S, Bonnici HM, Teki S, Agus TR, Pressnitzer D, Maguire EA, Griffiths TD (2014) Representations of specific acoustic patterns in the auditory cortex and hippocampus. P Roy Soc B Biol Sci. https://doi.org/10.1098/rspb.2014.1000
Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SCR, van der Kouwe AJW, Salat DH, Dale AM, Fischl B (2003) Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiat 60(9):878–888. https://doi.org/10.1001/archpsyc.60.9.878
Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G (2009) Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage 46(1):213–218. https://doi.org/10.1016/j.neuroimage.2009.01.069
Langer N, Hanggi J, Muller NA, Simmen HP, Jancke L (2012) Effects of limb immobilization on brain plasticity. Neurology 78(3):182–188. https://doi.org/10.1212/WNL.0b013e31823fcd9c
Langguth B, Zowe M, Landgrebe M, Sand P, Kleinjung T, Binder H, Hajak G, Eichhammer P (2006) Transcranial magnetic stimulation for the treatment of tinnitus: a new coil positioning method and first results. Brain Topogr 18(4):241–247. https://doi.org/10.1007/s10548-006-0002-1
Lanting CP, De Kleine E, Bartels H, Van Dijk P (2008) Functional imaging of unilateral tinnitus using fMRI. Acta Oto-Laryngol 128(4):415–421. https://doi.org/10.1080/00016480701793743
Lanting CP, de Kleine E, van Dijk P (2009) Neural activity underlying tinnitus generation: results from PET and fMRI. Hearing Res 255(1–2):1–13. https://doi.org/10.1016/j.heares.2009.06.009
Leaver AM, Seydell-Greenwald A, Turesky TK, Morgan S, Kim HJ, Rauschecker JP (2012) Cortico-limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci 6:21. https://doi.org/10.3389/fnsys.2012.00021
Lecluyse W, Meddis R (2009) A simple single-interval adaptive procedure for estimating thresholds in normal and impaired listeners. J Acoust Soc Am 126(5):2570–2579. https://doi.org/10.1121/1.3238248
Lecluyse W, Tan CM, McFerran D, Meddis R (2013) Acquisition of auditory profiles for good and impaired hearing. Int J Audiol 52(9):596–605. https://doi.org/10.3109/14992027.2013.796530
Lehericy S, Duffau H, Cornu P, Capelle L, Pidoux B, Carpentier A, Auliac S, Clemenceau S, Sichez JP, Bitar A, Valery CA, Van Effenterre R, Faillot T, Srour A, Fohanno D, Philippon J, Le Bihan D, Marsault C (2000) Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg 92(4):589–598. https://doi.org/10.3171/jns.2000.92.4.0589
Lehrl S (1977) Mehrfachwahl-wortschatz intelligenz test (MWT-B). Perimed, Erlangen
Lehrl SGA, Blaha L, Fischer B (1992) Kurztest fu ̈rallgemeine Intelligenz (KAI). Vless, Ebersberg
Lenarz T, Schreiner C, Snyder RL, Ernst A (1993) Neural mechanisms of tinnitus. Eur Arch Oto-Rhino-L 249(8):441–446
Lewis JW, Wightman FL, Brefczynski JA, Phinney RE, Binder JR, DeYoe EA (2004) Human brain regions involved in recognizing environmental sounds. Cereb Cortex 14(9):1008–1021. https://doi.org/10.1093/cercor/bhh061
Liebenthal E, Desai R, Ellingson MM, Ramachandran B, Desai A, Binder JR (2010) Specialization along the left superior temporal sulcus for auditory categorization. Cereb Cortex 20(12):2958–2970. https://doi.org/10.1093/cercor/bhq045
Liu YW, Lv H, Zhao PF, Liu ZH, Chen WJ, Gong SS, Wang ZC, Zhu JM (2018) Neuroanatomical alterations in patients with early stage of unilateral pulsatile tinnitus: a voxel-based morphometry study. Neural Plast. https://doi.org/10.1155/2018/4756471
Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP (1999) Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. P Natl Acad Sci USA 96(26):15222–15227. https://doi.org/10.1073/pnas.96.26.15222
McCormack A, Edmondson-Jones M, Fortnum H, Dawes P, Middleton H, Munro KJ, Moore DR (2014) The prevalence of tinnitus and the relationship with neuroticism in a middle-aged UK population. J Psychosom Res 76(1):56–60. https://doi.org/10.1016/j.jpsychores.2013.08.018
McKenna L, Handscomb L, Hoare DJ, Hall DA (2014) A scientific cognitive-behavioral model of tinnitus: novel conceptualizations of tinnitus distress. Front Neurol. https://doi.org/10.3389/fneur.2014.00196
Melcher JR, Sigalovsky IS, Guinan JJ, Levine RA (2000) Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol 83(2):1058–1072. https://doi.org/10.1152/jn.2000.83.2.1058
Messina A, Monda V, Avola R, Moscatelli F, Valenzano AA, Villano I, Ruberto M, Monda E, La Marra M, Tafuri D, Chieffi S, Cibelli G, Monda M, Messina G (2017) Role of the Orexin system on arousal, attention, feeding behaviour and sleep disorders. Acta Medica Mediterr 33(4):645–649. https://doi.org/10.19193/0393-6384_2017_4_096
Meyer M, Baumann S, Marchina S, Jancke L (2007) Hemodynamic responses in human multisensory and auditory association cortex to purely visual stimulation. Bmc Neurosci. https://doi.org/10.1186/1471-2202-8-14
Meyer M, Luethi MS, Neff P, Langer N, Buchi S (2014) Disentangling tinnitus distress and tinnitus presence by means of EEG power analysis. Neural Plast. https://doi.org/10.1155/2014/468546
Meyer M, Neff P, Liem F, Kleinjung T, Weidt S, Langguth B, Schecklmann M (2016) Differential tinnitus-related neuroplastic alterations of cortical thickness and surface area. Hearing Res 342:1–12. https://doi.org/10.1016/j.heares.2016.08.016
Moore DR, Edmondson-Jones M, Dawes P, Fortnum H, McCormack A, Pierzycki RH, Munro KJ (2014) Relation between speech-in-noise threshold, hearing loss and cognition from 40–69 years of age. Plos one. https://doi.org/10.1371/journal.pone.0107720
Morosan P, Schleicher A, Amunts K, Zilles K (2005) Multimodal architectonic mapping of human superior temporal gyrus. Anat Embryol 210(5–6):401–406. https://doi.org/10.1007/s00429-005-0029-1
Muhlau M, Rauschecker JP, Oestreicher E, Gaser C, Rottinger M, Wohlschlager AM, Simon F, Etgen T, Conrad B, Sander D (2006) Structural brain changes in tinnitus. Cereb Cortex 16(9):1283–1288. https://doi.org/10.1093/cercor/bhj070
Muller N, Keil J, Obleser J, Schulz H, Grunwald T, Bernays RL, Huppertz HJ, Weisz N (2013) You can’t stop the music: reduced auditory alpha power and coupling between auditory and memory regions facilitate the illusory perception of music during noise. Neuroimage 79:383–393. https://doi.org/10.1016/j.neuroimage.2013.05.001
Murray EA, Wise SP (2010) Interactions between orbital prefrontal cortex and amygdala: advanced cognition, learned responses and instinctive behaviors. Curr Opin Neurobiol 20(2):212–220. https://doi.org/10.1016/j.conb.2010.02.001
Naber PA, Witter MP, Da Silva FHL (2000) Networks of the hippocampal memory system of the rat - the pivotal role of the subiculum. Ann Ny Acad Sci 911:392–403
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Newman CW, Jacobson GP, Spitzer JB (1996) Development of the tinnitus handicap inventory. Arch Otolaryngol 122(2):143–148
Nichols T, Hayasaka S (2003) Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res 12(5):419–446. https://doi.org/10.1191/0962280203sm341ra
Nichols NR, Zieba M, Bye N (2001) Do glucocorticoids contribute to brain aging? Brain Res Rev 37(1–3):273–286. https://doi.org/10.1016/S0165-0173(01)00131-X
Okamoto H, Stracke H, Stoll W, Pantev C (2010) Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. P Natl Acad Sci USA 107(3):1207–1210. https://doi.org/10.1073/pnas.0911268107
Olsen RK, Moses SN, Riggs L, Ryan JD (2012) The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2012.00146
Oosterloo BC, Croll PH, de Jong RJB, Ikram MK, Goedegebure A (2021) Prevalence of tinnitus in an aging population and its relation to age and hearing loss. Otolaryng Head Neck 164(4):859–868. https://doi.org/10.1177/0194599820957296
Pessoa L (2011) Reprint of: emotion and cognition and the amygdala: from “what is it?” to “what’s to be done?” Neuropsychologia 49(4):681–694. https://doi.org/10.1016/j.neuropsychologia.2011.02.030
Pichora-Fuller MK, Souza PE (2003) Effects of aging on auditory processing of speech. Int J Audiol 42:S11–S16
Poeppel D (2003) The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time.” Speech Commun 41(1):245–255. https://doi.org/10.1016/S0167-6393(02)00107-3
Poppenk J, Evensmoen HR, Moscovitch M, Nadel L (2013) Long-axis specialization of the human hippocampus. Trends Cogn Sci 17(5):230–240. https://doi.org/10.1016/j.tics.2013.03.005
Profant O, Skoch A, Tintera J, Svobodova V, Kucharova D, Burianova JS, Syka J (2020) The influence of aging, hearing, and tinnitus on the morphology of cortical gray matter, amygdala, and hippocampus. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2020.553461
Rakic P (1995) A small step for the cell, a giant leap for mankind - a hypothesis of neocortical expansion during evolution. Trends Neurosci 18(9):383–388. https://doi.org/10.1016/0166-2236(95)93934-P
Rakic P (2000) Radial unit hypothesis of neocortical expansion. Novartis Found Symp 228:30–42. https://doi.org/10.1002/0470846631.ch3. (discussion 42-52)
Rasia-Filho AA, Londero RG, Achaval M (2000) Functional activities of the amygdala: an overview. J Psychiatr Neurosci 25(1):14–23
Rauschecker JP (2012) Ventral and dorsal streams in the evolution of speech and language. Front Evol Neurosci 4:7. https://doi.org/10.3389/fnevo.2012.00007
Rauschecker JP, Scott SK (2009) Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci 12(6):718–724. https://doi.org/10.1038/nn.2331
Rauschecker JP, Leaver AM, Muhlau M (2010) Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66(6):819–826. https://doi.org/10.1016/j.neuron.2010.04.032
R: A language and environment for statistical computing, R Foundation for Statistical Computing (2020). https://www.r-project.org/
Rodriguez-Fornells A, Cunillera T, Mestres-Misse A, de Diego-Balaguer R (2009) Neurophysiological mechanisms involved in language learning in adults. Philos Trans R Soc Lond B Biol Sci 364(1536):3711–3735. https://doi.org/10.1098/rstb.2009.0130
Roozendaal B, Nguyen BT, Power AE, McGaugh JL (1999) Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. P Natl Acad Sci USA 96(20):11642–11647. https://doi.org/10.1073/pnas.96.20.11642
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002) Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 58(5):695–701. https://doi.org/10.1212/Wnl.58.5.695
Rosing SN, Schmidt JH, Wedderkopp N, Baguley DM (2016) Prevalence of tinnitus and hyperacusis in children and adolescents: a systematic review. BMJ Open. https://doi.org/10.1136/bmjopen-2015-010596
Sahlsten H, Taiminen T, Karukivi M, Sjosten N, Nikkila J, Virtanen J, Paavola J, Joutsa J, Niinivirta-Joutsa K, Takala M, Holm A, Rauhala E, Loyttyniemi E, Johansson R, Jaaskelainen SK (2018) Psychiatric (Axis I) and personality (Axis II) disorders and subjective psychiatric symptoms in chronic tinnitus. Int J Audiol 57(4):302–312. https://doi.org/10.1080/14992027.2017.1409440
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B (2004) Thinning of the cerebral cortex in aging. Cereb Cortex 14(7):721–730. https://doi.org/10.1093/cercor/bhh032
Sander D, Grafman J, Zalla T (2003) The human amygdala: an evolved system for relevance detection. Rev Neurosci 14(4):303–316. https://doi.org/10.1515/Revneuro.2003.14.4.303
Sapolsky RM, Uno H, Rebert CS, Finch CE (1990) Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 10(9):2897–2902. https://doi.org/10.1523/jneurosci.10-09-02897.1990
Savage LM, Ramos RL (2009) Reward expectation alters learning and memory: the impact of the amygdala on appetitive-driven behaviors. Behav Brain Res 198(1):1–12. https://doi.org/10.1016/j.bbr.2008.10.028
Savastano M (2008) Tinnitus with or without hearing loss: are its characteristics different? Eur Arch Oto-Rhino-L 265(11):1295–1300. https://doi.org/10.1007/s00405-008-0630-z
Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, Stevens A, Van Leemput K, McKee A, Frosch MP, Fischl B, Augustinack JC, AsD N (2017) High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage 155:370–382. https://doi.org/10.1016/j.neuroimage.2017.04.046
Schecklmann M, Lehner A, Poeppl TB, Kreuzer PM, Rupprecht R, Rackl J, Burger J, Frank E, Hajak G, Langguth B, Landgrebe M (2013) Auditory cortex is implicated in tinnitus distress: a voxel-based morphometry study. Brain Struct Funct 218(4):1061–1070. https://doi.org/10.1007/s00429-013-0520-z
Schmidt SA, Zimmerman B, Medina ROB, Carpenter-Thompson JR, Husain FT (2018) Changes in gray and white matter in subgroups within the tinnitus population. Brain Res 1679:64–74. https://doi.org/10.1016/j.brainres.2017.11.012
Schmitt R, Meyer M, Giroud N (2022) Better speech-in-noise comprehension is associated with enhanced neural speech tracking in older adults with hearing impairment. Cortex 151:133–146. https://doi.org/10.1016/j.cortex.2022.02.017
Schneider P, Andermann M, Wengenroth M, Goebel R, Flor H, Rupp A, Diesch E (2009) Reduced volume of Heschl’s gyrus in tinnitus. Neuroimage 45(3):927–939. https://doi.org/10.1016/j.neuroimage.2008.12.045
Schonwiesner M, Rubsamen R, von Cramon DY (2005) Hemispheric asymmetry for spectral and temporal processing in the human antero-lateral auditory belt cortex. Eur J Neurosci 22(6):1521–1528. https://doi.org/10.1111/j.1460-9568.2005.04315.x
Schulze K, Gaab N, Schlaug G (2009) Perceiving pitch absolutely: comparing absolute and relative pitch possessors in a pitch memory task. Bmc Neurosci. https://doi.org/10.1186/1471-2202-10-106
Scott-Wittenborn N, Karadaghy OA, Piccirillo JF, Peelle JE (2017) A methodological assessment of studies that use voxel-based morphometry to study neural changes in tinnitus patients. Hearing Res 355:23–32. https://doi.org/10.1016/j.heares.2017.09.002
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosur Ps 20(1):11–21. https://doi.org/10.1136/jnnp.20.1.11
Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004) A hybrid approach to the skull stripping problem in MRI. Neuroimage 22(3):1060–1075. https://doi.org/10.1016/j.neuroimage.2004.03.032
Seydell-Greenwald A, Leaver AM, Turesky TK, Morgan S, Kim HJ, Rauschecker JP (2012) Functional MRI evidence for a role of ventral prefrontal cortex in tinnitus. Brain Res 1485:22–39. https://doi.org/10.1016/j.brainres.2012.08.052
Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, Sunaert S (2007) Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology 49(8):669–679. https://doi.org/10.1007/s00234-007-0231-3
Snyder JS, Alain C (2007) Toward a neurophysiological theory of auditory stream segregation. Psychol Bull 133(5):780–799. https://doi.org/10.1037/0033-2909.133.5.780
Specht K, Wigglesworth P (2018) The functional and structural asymmetries of the superior temporal sulcus. Scand J Psychol 59(1):74–82. https://doi.org/10.1111/sjop.12410
Squire LR, Zolamorgan S (1991) The medial temporal-lobe memory system. Science 253(5026):1380–1386. https://doi.org/10.1126/science.1896849
Tae WS, Yakunina N, Lee WH, Ryu YJ, Ham HK, Pyun SB, Nam EC (2018) Changes in the regional shape and volume of subcortical nuclei in patients with tinnitus comorbid with mild hearing loss. Neuroradiology 60(11):1203–1211. https://doi.org/10.1007/s00234-018-2093-2
Tata DA, Anderson BJ (2010) The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol Behav 99(2):186–193. https://doi.org/10.1016/j.physbeh.2009.09.008
Tian X, Zarate JM, Poeppel D (2016) Mental imagery of speech implicates two mechanisms of perceptual reactivation. Cortex 77:1–12. https://doi.org/10.1016/j.cortex.2016.01.002
Urgolites ZJ, Wixted JT, Goldinger SD, Papesh MH, Treiman DM, Squire LR, Steinmetz PN (2022) Two kinds of memory signals in neurons of the human hippocampus. P Natl Acad Sci USA. https://doi.org/10.1073/pnas.2115128119
van der Meer D, Kaufmann T (2022) Mapping the genetic architecture of cortical morphology through neuroimaging: progress and perspectives. Transl Psychiat. https://doi.org/10.1038/s41398-022-02193-5
Vanneste S, Van de Heyning P, De Ridder D (2015) Tinnitus: a large VBM-EEG correlational study. Plos one. https://doi.org/10.1371/journal.pone.0115122
Vielsmeier V, Stiel RS, Kwok PL, Langguth B, Schecklmann M (2020) From acute to chronic tinnitus: pilot data on predictors and progression. Front Neurol. https://doi.org/10.3389/fneur.2020.00997
Wagener K, Brand T, Kollmeier B (1999) Entwicklung und evaluation eines satztests für die deutsche sprache teil III: optimierung des oldenburger satztests. Z Audiol. https://doi.org/10.1039/C4LC00330F
Wallhausser-Franke E, D’Amelio R, Glauner A, Delb W, Servais JJ, Hormann K, Repik I (2017) Transition from acute to chronic tinnitus: predictors for the development of chronic distressing tinnitus. Front Neurol. https://doi.org/10.3389/fneur.2017.00605
Wang Q, Verweij EWE, Krugers HJ, Joels M, Swaab DF, Lucassen PJ (2014) Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Struct Funct 219(5):1615–1626. https://doi.org/10.1007/s00429-013-0589-4
Wang SF, Ritchey M, Libby LA, Ranganath C (2016) Functional connectivity based parcellation of the human medial temporal lobe. Neurobiol Learn Mem 134:123–134. https://doi.org/10.1016/j.nlm.2016.01.005
Wasserthal C, Brechmann A, Stadler J, Fischl B, Engel K (2014) Localizing the human primary auditory cortex in vivo using structural MRI. Neuroimage 93:237–251. https://doi.org/10.1016/j.neuroimage.2013.07.046
Wechsler D (1981) The psychometric tradition - developing the wechsler adult intelligence scale. Contemp Educ Psychol 6(2):82–85. https://doi.org/10.1016/0361-476x(81)90035-7
Wei X, Lv H, Chen Q, Wang ZD, Liu CL, Zhao PF, Gong SS, Yang ZH, Wang ZC (2021) Cortical thickness alterations in patients with tinnitus before and after sound therapy: a surface-based morphometry study. Front Neurosci-Switz. https://doi.org/10.3389/fnins.2021.633364
Weisz N, Hartmann T, Dohrmann K, Schlee W, Norena A (2006) High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear Res 222(1–2):108–114. https://doi.org/10.1016/j.heares.2006.09.003
Werner S, Noppeney U (2010) Superadditive responses in superior temporal sulcus predict audiovisual benefits in object categorization. Cereb Cortex 20(8):1829–1842. https://doi.org/10.1093/cercor/bhp248
Wisse LE, Biessels GJ, Geerlings MI (2014) A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front Aging Neurosci 6:261. https://doi.org/10.3389/fnagi.2014.00261
Woollett K, Maguire EA (2011) Acquiring “the Knowledge” of London’s layout drives structural brain changes. Curr Biol 21(24):2109–2114. https://doi.org/10.1016/j.cub.2011.11.018
Zaitchik D, Walker C, Miller S, LaViolette P, Feczko E, Dickerson BC (2010) Mental state attribution and the temporoparietal junction: an fMRI study comparing belief, emotion, and perception. Neuropsychologia 48(9):2528–2536. https://doi.org/10.1016/j.neuropsychologia.2010.04.031
Zald DH (2003) The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev 41(1):88–123. https://doi.org/10.1016/S0165-0173(02)00248-5
Zatorre RJ, Belin P (2001) Spectral and temporal processing in human auditory cortex. Cereb Cortex 11(10):946–953. https://doi.org/10.1093/cercor/11.10.946
Zhang LQ, Wu C, Martel DT, West M, Sutton MA, Shore SE (2019) Remodeling of cholinergic input to the hippocampus after noise exposure and tinnitus induction in Guinea pigs. Hippocampus 29(8):669–682. https://doi.org/10.1002/hipo.23058
Zhang LQ, Wang JJ, Sun HY, Feng GD, Gao ZQ (2022) Interactions between the hippocampus and the auditory pathway. Neurobiol Learn Mem. https://doi.org/10.1016/j.nlm.2022.107589
Zilles K, Armstrong E, Schleicher A, Kretschmann HJ (1988) The human pattern of gyrification in the cerebral-cortex. Anat Embryol 179(2):173–179. https://doi.org/10.1007/Bf00304699
Acknowledgements
This research was supported by the Swiss National Science Foundation (project numbers 105319–169964 and 105314–152905 to MM), “Schwerhörigen-Verein Nordwestschweiz” and the “Zürcher Stiftung für das Hören”.
Funding
Open access funding provided by University of Zurich. This work was supported by Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung with grant number (105319–169964).
Author information
Authors and Affiliations
Contributions
MM and NG: planned the study. RS: performed the MRI measurements and computed the morphometric analyses. SE: performed the statistical analyses of the demographic, audiometric and psychometric data. SE: wrote the original draft of the manuscript. RS, NG and MM: contributed to the interpretation of the data and to the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elmer, S., Schmitt, R., Giroud, N. et al. The neuroanatomical hallmarks of chronic tinnitus in comorbidity with pure-tone hearing loss. Brain Struct Funct 228, 1511–1534 (2023). https://doi.org/10.1007/s00429-023-02669-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-023-02669-0