Abstract
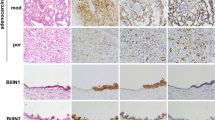
Bile duct biopsy is being increasingly performed in number for a definite diagnosis of cholangiocarcinoma. However, difficulties are associated with a histopathological diagnosis because of the limited small amount of specimen obtained and crash artifact. The aim of the present study was to identify useful diagnostic immunohistochemical markers in bile duct biopsy that support a histological diagnosis. Fifty-one bile duct biopsy samples, including 26 samples taken from patients with cholangiocarcinoma, 11 with intraductal papillary neoplasm of the bile duct (IPNB), and 14 with benign bile duct lesions, were examined. Histology and the immunohistochemical expression of insulin-like growth factor II mRNA-binding protein 3 (IMP3), enhancer of zeste homolog 2 (EZH2), and p53 were assessed. They were then evaluated for their usefulness as diagnostic markers of malignancy. The diagnostic sensitivity and accuracy of the institutional histological diagnosis were 53.8% and 70.0%, respectively. The diagnostic sensitivity and accuracy of IMP3, EZH2, and p53 were 69.2% and 80.0%, 76.9% and 85.0%, and 50.0% and 67.5%, respectively. Immunohistochemical staining for EZH2; the combination of either 2 of IMP3, EZH2, and p53; or the combination of IMP3, EZH2, and p53 significantly increased sensitivity and accuracy over those of the institutional histological diagnosis (p<0.05). In conclusion, an immunohistochemical panel consisting of IMP3, EZH2, and p53 increases the diagnostic sensitivity and accuracy of bile duct biopsy for the diagnosis of cholangiocarcinoma.


Similar content being viewed by others
Data availability
All data generated or analyzed during the present study were included in the published article.
Code availability
Not applicable.
References
Dumonceau JM, Delhaye M, Charette N, Farina A (2020) Challenging biliary strictures: pathophysiological features, differential diagnosis, diagnostic algorithms, and new clinically relevant biomarkers - part 1. Ther Adv Gastroenterol 13:1756284820927292. https://doi.org/10.1177/1756284820927292
Kawashima H, Itoh A, Ohno E, Miyahara R, Ohmiya N, Tanaka T, Shimoyama Y, Nakamura S, Ebata T, Nagino M, Goto H, Hirooka Y (2013) Diagnostic and prognostic value of immunohistochemical expression of S100P and IMP3 in transpapillary biliary forceps biopsy samples of extrahepatic bile duct carcinoma. J Hepatobiliary Pancreat Sci 20:441–447. https://doi.org/10.1007/s00534-012-0581-z
Singhi AD, Nikiforova MN, Chennat J, Papachristou GI, Khalid A, Rabinovitz M, Das R, Sarkaria S, Ayasso MS, Wald AI, Monaco SE, Nalesnik M, Ohori NP, Geller D, Tsung A, Zureikat AH, Zeh H, Marsh JW, Hogg M, Lee K, Bartlett DL, Pingpank JF, Humar A, Bahary N, Dasyam AK, Brand R, Fasanella KE, McGrath K, Slivka A (2020) Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 69:52–61. https://doi.org/10.1136/gutjnl-2018-317817
De Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L Jr, Watkins JL, Lehman GA (2002) Tissue sampling at ERCP in suspected malignant biliary strictures (part 1). Gastrointest Endosc 56:552–561. https://doi.org/10.1067/mge.2002.128132
de Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L Jr, Watkins JL, Lehman GA (2002) Tissue sampling at ERCP in suspected malignant biliary strictures (part 2). Gastrointest Endosc 56:720–730. https://doi.org/10.1067/mge.2002.129219
Levy M, Lin F, Xu H, Dhall D, Spaulding BO, Wang HL (2010) S100P, von Hippel-Lindau gene product, and IMP3 serve as a useful immunohistochemical panel in the diagnosis of adenocarcinoma on endoscopic bile duct biopsy. Hum Pathol 41:1210–1219. https://doi.org/10.1016/j.humpath.2010.01.014
Sasaki M, Sato Y (2017) Insulin-like growth factor II mRNA-binding protein 3 (IMP3) is a marker that predicts presence of invasion in papillary biliary tumors. Hum Pathol 62:152–159. https://doi.org/10.1016/j.humpath.2016.12.028
Gao Y, Yang M, Jiang Z, Woda BA, Mercurio AM, Qin J, Huang X, Zhang F (2014) IMP3 expression is associated with poor outcome and epigenetic deregulation in intrahepatic cholangiocarcinoma. Hum Pathol 45:1184–1191. https://doi.org/10.1016/j.humpath.2014.01.016
Morimatsu K, Aishima S, Yamamoto H, Hayashi A, Nakata K, Oda Y, Shindo K, Fujino M, Tanaka M, Oda Y (2013) Insulin-like growth factor II messenger RNA-binding protein-3 is a valuable diagnostic and prognostic marker of intraductal papillary mucinous neoplasm. Hum Pathol 44:1714–1721. https://doi.org/10.1016/j.humpath.2012.12.020
Peng DF, Kanai Y, Sawada M, Ushijima S, Hiraoka N, Kitazawa S, Hirohashi S (2006) DNA methylation of multiple tumor-related genes in association with overexpression of DNA methyltransferase 1 (DNMT1) during multistage carcinogenesis of the pancreas. Carcinogenesis 27:1160–1168. https://doi.org/10.1093/carcin/bgi361
Riener MO, Fritzsche FR, Clavien PA, Pestalozzi BC, Probst-Hensch N, Jochum W, Kristiansen G (2009) IMP3 expression in lesions of the biliary tract: a marker for high-grade dysplasia and an independent prognostic factor in bile duct carcinomas. Hum Pathol 40:1377–1383. https://doi.org/10.1016/j.humpath.2009.01.024
Saito Y, Kanai Y, Nakagawa T, Sakamoto M, Saito H, Ishii H, Hirohashi S (2003) Increased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int J Cancer 105:527–532. https://doi.org/10.1002/ijc.11127
Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M (1999) The oncogene and polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397:164–168
Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M (2004) Stem cells and cancer; the polycomb connection. Cell 118:409–418
Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM (2003) EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 100:11606–11611
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM (2002) The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419:624–629
Sasaki M, Matsubara T, Yoneda N, Nomoto K, Tsuneyama K, Sato Y, Nakanuma Y (2013) Overexpression of enhancer of zeste homolog 2 and MUC1 may be related to malignant behaviour in intraductal papillary neoplasm of the bile duct. Histopathology 62:446–457. https://doi.org/10.1111/his.12016
Nakanuma Y, Curado M, Fransceschi S, Gores G, Paradis V, Sripa B, Tsui W, Wee S (2010) Intrahepatic cholagiocarcinoma. In: Bosman F, Carneiro F, Hruban H, Theise N (eds) WHO classification of tumours of the digestive system, 4th edn. IARC, Lyon, pp 217–227
Sasaki M, Nakanuma Y, Kim Y (1996) Characterization of apomicin expression in intrahepatic cholangio-carcinomas and their precursor lesions: an immunohistochemical study. Hepatology 24:1074–1078
Sasaki M, Yamaguchi J, Itatsu K, Ikeda H, Nakanuma Y (2008) Over-expression of polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J Pathol 215:175–183
Yamaguchi J, Sasaki M, Harada K, Zen Y, Sato Y, Ikeda H, Itatsu K, Yokoyama Y, Ando H, Ohta T, Kubota A, Shimizu K, Nimura Y, Nagino M, Nakanuma Y (2009) Papillary hyperplasia of the gallbladder in pancreaticobiliary maljunction represents a senescence-related lesion induced by lysolecithin. Lab Investig 89:1018–1031. https://doi.org/10.1038/labinvest.2009.65
Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y (2006) Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct--an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol 44:350–358
Hsu M, Sasaki M, Igarashi S, Sato Y, Nakanuma Y (2013) KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer 119:1669–1674. https://doi.org/10.1002/cncr.27955
Nakanuma Y, Sasaki M, Sato Y, Ren X, Ikeda H, Harada K (2009) Multistep carcinogenesis of perihilar cholangiocarcinoma arising in the intrahepatic large bile ducts. World J Hepatol 1:35–42. https://doi.org/10.4254/wjh.v1.i1.35
Wang HL, Kim CJ, Koo J, Zhou W, Choi EK, Arcega R, Chen ZE, Wang H, Zhang L, Lin F (2017) Practical immunohistochemistry in neoplastic pathology of the gastrointestinal tract, liver, biliary tract, and pancreas. Arch Pathol Lab Med 141:1155–1180. https://doi.org/10.5858/arpa.2016-0489-RA
Kobel M, Reuss A, du Bois A, Kommoss S, Kommoss F, Gao D, Kalloger SE, Huntsman DG, Gilks CB (2010) The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. J Pathol 222:191–198. https://doi.org/10.1002/path.2744
Burnett AS, Quinn PL, Ajibade DV, Peters SR, Ahlawat SK, Mahmoud OM, Chokshi RJ (2019) Design of an immunohistochemistry biomarker panel for diagnosis of pancreatic adenocarcinoma. Pancreatology 19:842–849. https://doi.org/10.1016/j.pan.2019.08.007
Senoo J, Mikata R, Kishimoto T, Hayashi M, Kusakabe Y, Yasui S, Yamato M, Ohyama H, Sugiyama H, Tsuyuguchi T, Yoshitomi H, Ohtsuka M, Maeda J, Ota S, Nakatani Y, Kato N (2018) Immunohistochemical analysis of IMP3 and p53 expression in endoscopic ultrasound-guided fine needle aspiration and resected specimens of pancreatic diseases. Pancreatology 18:176–183. https://doi.org/10.1016/j.pan.2017.12.010
Funding
The present study was supported in part by a Grant-in Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports and Science and Technology of Japan (18K06985).
Author information
Authors and Affiliations
Contributions
Study conception and design were performed by Motoko Sasaki. Material preparation and data collection and analysis were performed by Motoko Sasaki and Yasunori Sato. The first draft of the manuscript was written by Motoko Sasaki and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The Ethics Committee of Kanazawa University approved the present study. The work described herein was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent to participate
Informed consent was obtained for experimentation with human subjects.
Consent for publication
Informed consent was obtained for experimentation with human subjects.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sasaki, M., Sato, Y. An immunohistochemical panel of insulin-like growth factor II mRNA-binding protein 3 (IMP3), enhancer of zeste homolog 2 (EZH2), and p53 is useful for a diagnosis in bile duct biopsy. Virchows Arch 479, 697–703 (2021). https://doi.org/10.1007/s00428-021-03132-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03132-3