Abstract
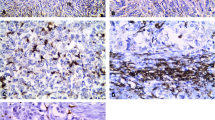
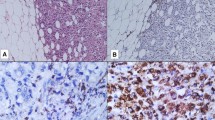
Immune response can affect tumour progression and treatment outcome. This study investigated the potential of stromal macrophages around ductal carcinoma in situ (DCIS) in predicting recurrence and progression. CD68 and CD163 expression of macrophages in DCIS from 198 patients was determined by immunohistochemistry. Disease free survival (DFS), clinicopathological parameters and biomarker expression were correlated with the densities of both CD68+ and CD163+ macrophages. High CD68+ macrophage density was associated with high nuclear grade (p < 0.001), oestrogen receptor (ER) negativity (p = 0.029), progesterone receptor (PR) negativity (p = 0.008) and human epidermal growth factor receptor 2 (HER2) positivity (p < 0.001). High CD163+ macrophage density was associated with high nuclear grade (p = 0.003), microinvasion (p = 0.01), ER negativity (p < 0.001), PR negativity (p = 0.001), HER2 positivity (p = 0.001) and triple negativity (p = 0.022). DCIS with higher CD68+ macrophage density disclosed significantly worse DFS for ipsilateral invasive recurrence (p = 0.004) and is affirmed by multivariate Cox regression analysis (95% CI 1.126–5.102, HR = 2.397, p = 0.023). DCIS with higher CD163+ macrophage density showed significantly worse DFS for both recurrence (p = 0.001) and ipsilateral invasive recurrence (p = 0.001). These findings, for CD163+ macrophage density, were affirmed by multivariate Cox regression analysis respectively for both recurrence (95% CI 1.210–2.293, HR = 1.880, p = 0.005) and ipsilateral invasive recurrence (95% CI 1.122–5.176, HR = 2.410, p = 0.024). This study demonstrated that DCIS with higher macrophage density was associated with poorer prognostic parameters, while DCIS with higher CD163+ macrophage density predicted both recurrence and ipsilateral invasive recurrence.

Similar content being viewed by others
References
Koh VC, Lim JC, Thike AA, Cheok PY, Thu MM, Tan VK, Tan BK, Ong KW, Ho GH, Tan WJ, Tan Y, Salahuddin AS, Busmanis I, Chong AP, Iqbal J, Thilagaratnam S, Wong JS, Tan PH (2015) Characteristics and behaviour of screen-detected ductal carcinoma in situ of the breast: comparison with symptomatic patients. Breast Cancer Res Treat 152(2):293–304. https://doi.org/10.1007/s10549-015-3472-6
Lee RJ, Vallow LA, McLaughlin SA, Tzou KS, Hines SL, Peterson JL (2012) Ductal carcinoma in situ of the breast. Int J Surg Oncol 2012:12. https://doi.org/10.1155/2012/123549
Jara-Lazaro AR, Thilagaratnam S, Tan PH (2010) Breast cancer in Singapore: some perspectives. Breast Cancer (Tokyo, Japan) 17(1):23–28. https://doi.org/10.1007/s12282-009-0155-3
Tan PH, Chiang GS, Ng EH, Low SC, Ng FC (1999) Screen detected breast cancer in an Asian population: pathological findings of the Singapore breast screening project. Breast (Edinburgh, Scotland) 8(3):120–125. https://doi.org/10.1054/brst.1999.0038
Tan PH, Chuah KL, Chiang G, Wong CY, Dong F, Bay BH (2002) Correlation of p53 and cerbB2 expression and hormonal receptor status with clinicopathologic parameters in ductal carcinoma in situ of the breast. Oncol Rep 9(5):1081–1086
Pinder SE, Duggan C, Ellis IO, Cuzick J, Forbes JF, Bishop H, Fentiman IS, George WD (2010) A new pathological system for grading DCIS with improved prediction of local recurrence: results from the UKCCCR/ANZ DCIS trial. Br J Cancer 103(1):94–100. https://doi.org/10.1038/sj.bjc.6605718
Bobrow LG, Happerfield LC, Gregory WM, Millis RR (1995) Ductal carcinoma in situ: assessment of necrosis and nuclear morphology and their association with biological markers. J Pathol 176(4):333–341. https://doi.org/10.1002/path.1711760404
Mommers EC, Page DL, Dupont WD, Schuyler P, Leonhart AM, Baak JP, Meijer CJ, van Diest PJ (2001) Prognostic value of morphometry in patients with normal breast tissue or usual ductal hyperplasia of the breast. Int J Cancer 95(5):282–285
Nofech-Mozes S, Spayne J, Rakovitch E, Hanna W (2005) Prognostic and predictive molecular markers in DCIS: a review. Adv Anat Pathol 12(5):256–264
Shoker BS, Sloane JP (1999) DCIS grading schemes and clinical implications. Histopathology 35(5):393–400
Curiel TJ (2008) Regulatory T cells and treatment of cancer. Curr Opin Immunol 20(2):241–246. https://doi.org/10.1016/j.coi.2008.04.008
Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, Shin SW, Kim YH, Kim JS, Park KH (2013) Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol 36(3):224–231. https://doi.org/10.1097/COC.0b013e3182467d90
Knopfelmacher A, Fox J, Lo Y, Shapiro N, Fineberg S (2015) Correlation of histopathologic features of ductal carcinoma in situ of the breast with the oncotype DX DCIS score. Mod Pathol 28(9):1167–1173. https://doi.org/10.1038/modpathol.2015.79
Lal A, Chan L, Devries S, Chin K, Scott GK, Benz CC, Chen YY, Waldman FM, Hwang ES (2013) FOXP3-positive regulatory T lymphocytes and epithelial FOXP3 expression in synchronous normal, ductal carcinoma in situ, and invasive cancer of the breast. Breast Cancer Res Treat 139(2):381–390. https://doi.org/10.1007/s10549-013-2556-4
Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA (2014) The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat 148(3):467–476. https://doi.org/10.1007/s10549-014-3185-2
Ostuni R, Kratochvill F, Murray PJ, Natoli G (2015) Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol 36(4):229–239. https://doi.org/10.1016/j.it.2015.02.004
Campbell MJ, Baehner F, O'Meara T, Ojukwu E, Han B, Mukhtar R, Tandon V, Endicott M, Zhu Z, Wong J, Krings G, Au A, Gray JW, Esserman L (2017) Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res Treat 161(1):17–28. https://doi.org/10.1007/s10549-016-4036-0
Thompson E, Taube JM, Elwood H, Sharma R, Meeker A, Warzecha HN, Argani P, Cimino-Mathews A, Emens LA (2016) The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol 29(3):249–258. https://doi.org/10.1038/modpathol.2015.158
Casey T, Bond J, Tighe S, Hunter T, Lintault L, Patel O, Eneman J, Crocker A, White J, Tessitore J, Stanley M, Harlow S, Weaver D, Muss H, Plaut K (2009) Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Res Treat 114(1):47–62. https://doi.org/10.1007/s10549-008-9982-8
Yeong J, Thike AA, Tan PH, Iqbal J (2017) Identifying progression predictors of breast ductal carcinoma in situ. J Clin Pathol 70(2):102–108. https://doi.org/10.1136/jclinpath-2016-204154
Celis JE, Gromova I, Cabezon T, Gromov P, Shen T, Timmermans-Wielenga V, Rank F, Moreira JM (2007) Identification of a subset of breast carcinomas characterized by expression of cytokeratin 15: relationship between CK15+ progenitor/amplified cells and pre-malignant lesions and invasive disease. Mol Oncol 1(3):321–349. https://doi.org/10.1016/j.molonc.2007.09.004
Hannemann J, Velds A, Halfwerk JB, Kreike B, Peterse JL, van de Vijver MJ (2006) Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res 8(5):R61. https://doi.org/10.1186/bcr1613
Teo NB, Shoker BS, Jarvis C, Martin L, Sloane JP, Holcombe C (2003) Angiogenesis and invasive recurrence in ductal carcinoma in situ of the breast. Eur J Cancer 39(1):38–44
Rice A, Quinn CM (2002) Angiogenesis, thrombospondin, and ductal carcinoma in situ of the breast. J Clin Pathol 55(8):569–574
Whiteside T (2013) Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol 3:107. https://doi.org/10.3389/fonc.2013.00107
Chen XY, Yeong J, Thike AA, Bay BH, Tan PH (2019) Prognostic role of immune infiltrates in breast ductal carcinoma in situ. Breast Cancer Res Treat 177:17–27. https://doi.org/10.1007/s10549-019-05272-2
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. https://doi.org/10.1038/nature07205
Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, Anders RA, Kelly RJ (2017) Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 66(5):794–801. https://doi.org/10.1136/gutjnl-2015-310839
Eiró N, Pidal I, Fernandez-Garcia B, Junquera S, Lamelas ML, del Casar JM, González LO, López-Muñiz A, Vizoso FJ (2012) Impact of CD68/(CD3+CD20) ratio at the invasive front of primary tumors on distant metastasis development in breast cancer. PLoS One 7(12):e52796. https://doi.org/10.1371/journal.pone.0052796
Lee AH, Happerfield LC, Bobrow LG, Millis RR (1997) Angiogenesis and inflammation in ductal carcinoma in situ of the breast. J Pathol 181(2):200–206. https://doi.org/10.1002/(sici)1096-9896(199702)181:2<200::Aid-path726>3.0.Co;2-k
Lee AH, Dublin EA, Bobrow LG (1999) Angiogenesis and expression of thymidine phosphorylase by inflammatory and carcinoma cells in ductal carcinoma in situ of the breast. J Pathol 187(3):285–290. https://doi.org/10.1002/(sici)1096-9896(199902)187:3<285::Aid-path238>3.0.Co;2-r
Almatroodi SA, McDonald CF, Darby IA, Pouniotis DS (2016) Characterization of M1/M2 tumour-associated macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with NSCLC. Cancer Microenviron 9(1):1–11. https://doi.org/10.1007/s12307-015-0174-x
Aras S, Zaidi MR (2017) TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer 117:1583–1591. https://doi.org/10.1038/bjc.2017.356
Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G (2013) Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One 8(11):e80908. https://doi.org/10.1371/journal.pone.0080908
Jaguin M, Houlbert N, Fardel O, Lecureur V (2013) Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol 281(1):51–61. https://doi.org/10.1016/j.cellimm.2013.01.010
Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, Kindt A, Van den Bergh R, Leenen PJ, De Baetselier P, Ghassabeh GH (2005) Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol 77(3):321–327. https://doi.org/10.1189/jlb.0304212
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11(10):889–896. https://doi.org/10.1038/ni.1937
Tang X (2013) Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett 332(1):3–10. https://doi.org/10.1016/j.canlet.2013.01.024
Sica A, Saccani A, Mantovani A (2002) Tumor-associated macrophages: a molecular perspective. Int Immunopharmacol 2(8):1045–1054
Sakai Y, Honda M, Fujinaga H, Tatsumi I, Mizukoshi E, Nakamoto Y, Kaneko S (2008) Common transcriptional signature of tumor-infiltrating mononuclear inflammatory cells and peripheral blood mononuclear cells in hepatocellular carcinoma patients. Cancer Res 68(24):10267–10279. https://doi.org/10.1158/0008-5472.Can-08-0911
Holness C, Simmons D (1993) Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 81(6):1607–1613
Medrek C, Pontén F, Jirström K, Leandersson K (2012) The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12:306–306. https://doi.org/10.1186/1471-2407-12-306
Mahmoud SMA, Lee AHS, Paish EC, Macmillan RD, Ellis IO, Green AR (2012) Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 65(2):159–163. https://doi.org/10.1136/jclinpath-2011-200355
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4(1):71–78. https://doi.org/10.1038/nrc1256
Alkhateeb AA, Han B, Connor JR (2013) Ferritin stimulates breast cancer cells through an iron-independent mechanism and is localized within tumor-associated macrophages. Breast Cancer Res Treat 137(3):733–744. https://doi.org/10.1007/s10549-012-2405-x
Gatenbee C, West J, Baker AM, Guljar N, Jones L, Graham TA, Robertson-Tessi M, Anderson ARA (2019) Macrophage-mediated immunoediting drives ductal carcinoma evolution: space is the game changer. bioRxiv:594598. doi:https://doi.org/10.1101/594598
Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, Lisanti MP (2010) Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 9(16):3256–3276. https://doi.org/10.4161/cc.9.16.12553
Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, Pavlides S, Whitaker-Menezes D, Tsirigos A, Witkiewicz A, Lin Z, Balliet R, Howell A, Sotgia F (2010) Understanding the "lethal" drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther 10(6):537–542. https://doi.org/10.4161/cbt.10.6.13370
Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Caro J, Lisanti MP, Sotgia F (2010) Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle 9(17):3515–3533. https://doi.org/10.4161/cc.9.17.12928
Bae JY, Ahn SJ, Han W, Noh DY (2007) Peroxiredoxin I and II inhibit H2O2-induced cell death in MCF-7 cell lines. J Cell Biochem 101(4):1038–1045. https://doi.org/10.1002/jcb.21155
Ding J, Guo C, Hu P, Chen J, Liu Q, Wu X, Cao Y, Wu J (2016) CSF1 is involved in breast cancer progression through inducing monocyte differentiation and homing. Int J Oncol 49(5):2064–2074. https://doi.org/10.3892/ijo.2016.3680
Sousa S, Brion R, Lintunen M, Kronqvist P, Sandholm J, Mönkkönen J, Kellokumpu-Lehtinen P-L, Lauttia S, Tynninen O, Joensuu H, Heymann D, Määttä JA (2015) Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res 17(1):101. https://doi.org/10.1186/s13058-015-0621-0
Bogels M, Braster R, Nijland PG, Gul N, van de Luijtgaarden W, Fijneman RJ, Meijer GA, Jimenez CR, Beelen RH, van Egmond M (2012) Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunology 1(6):798–809. https://doi.org/10.4161/onci.20427
Shabo I, Stal O, Olsson H, Dore S, Svanvik J (2008) Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer 123(4):780–786. https://doi.org/10.1002/ijc.23527
Sumer H, Nicholls C, Pinto AR, Indraharan D, Liu J, Lim ML, Liu JP, Verma PJ (2010) Chromosomal and telomeric reprogramming following ES-somatic cell fusion. Chromosoma 119(2):167–176. https://doi.org/10.1007/s00412-009-0245-1
Rappa G, Mercapide J, Lorico A (2012) Spontaneous formation of tumorigenic hybrids between breast cancer and multipotent stromal cells is a source of tumor heterogeneity. Am J Pathol 180(6):2504–2515. https://doi.org/10.1016/j.ajpath.2012.02.020
Ding J, Jin W, Chen C, Shao Z, Wu J (2012) Tumor associated macrophage x cancer cell hybrids may acquire cancer stem cell properties in breast cancer. PLoS One 7(7):e41942. https://doi.org/10.1371/journal.pone.0041942
Funding
This study was supported by the SingHealth Foundation (SHF) Research Grant, SHF/FG668S/2015, awarded to Dr. Aye Aye Thike.
Author information
Authors and Affiliations
Contributions
AT conceived and directed the study. AT, PT and BB supervised the research. VK prepared tissue sections, performed IHC and collated data. AT and NM performed immunohistochemical scoring. AT and XC interpreted data and performed biostatistical analysis. PT and BB contributed to the scientific content of the study. XC drafted the manuscript with the assistance and final approval of all authors.
Corresponding author
Ethics declarations
This study was approved by The SingHealth Centralized Institutional Review Board (CIRB), Ref No. 2016/2393.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, XY., Thike, A.A., Md Nasir, N.D. et al. Higher density of stromal M2 macrophages in breast ductal carcinoma in situ predicts recurrence. Virchows Arch 476, 825–833 (2020). https://doi.org/10.1007/s00428-019-02735-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02735-1