Abstract
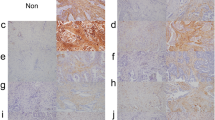
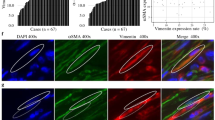
Cancer-associated fibroblasts (CAFs), activated fibroblasts in a cancer microenvironment, exert various effects upon carcinoma cells including lung adenocarcinoma cells. Various markers identifying CAFs have been proposed, but the correlations among these markers proposed and their clinicopathological significance have remained largely unknown. Therefore, in this study, we immunohistochemically evaluated the expression of alpha-smooth muscle actin (α-SMA), podoplanin, and periostin among these proposed markers in 92 cases of lung adenocarcinoma. These three markers were weakly correlated, but the relative abundance of α-SMA was significantly associated with high Ki-67 labelling index (LI), lymph node metastasis, and low 5-year overall survival (OS) rate of the patients. That of podoplanin was significantly associated with high pT and Ki-67 LI, distant metastasis, and low 5-year OS rate and that of periostin with high pT and Ki-67 LI. We then tentatively subclassified these cases into four groups according to high or low status of each of paired markers: α-SMA/podoplanin, α-SMA/periostin, and periostin/podoplanin. The α-SMA high/podoplanin high group was associated with the lowest survival rate (53.3%) among the four groups with significance. However, there were no significant differences in overall survival when the patients were classified according to the combinations of α-SMA/periostin or periostin/podoplanin. Results of our study firstly revealed the heterogeneity of CAFs in human lung adenocarcinoma tissue, and the analysis employing multiple markers of CAFs is generally required to study the clinical significance of CAFs in clinical materials.



Similar content being viewed by others
References
Mahale J, Smagurauskaite G, Brown K, Thomas A, Howells LM (2016) The role of stromal fibroblasts in lung carcinogenesis: a target for chemoprevention? Int J Cancer 138:30–44. https://doi.org/10.1002/ijc.29447
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598
Togo S, Polanska UM, Horimoto Y, Orimo A (2013) Carcinoma-associated fibroblasts are a promising therapeutic target. Cancers (Basel) 5:149–169
Kim S-H, Choe C, Shin Y-S, Jeon M-J, Choi S-J, Lee J, Bae GY, Cha HJ, Kim J (2013) Human lung cancer-associated fibroblasts enhance motility of non-small cell lung cancer cells in co-culture. Anticancer Res 33:2001–2009
Horie M, Saito A, Mikami Y, Ohshima M, Morishita Y, Nakajima J, Kohyama T, Nagase T (2012) Characterization of human lung cancer-associated fibroblasts in three-dimensional in vitro co-culture model. Biochem Biophys Res Commun 423:158–163
Chen Y, Zou L, Zhang Y, Chen Y, Xing P, Yang W, Li F, Ji X, Liu F, Lu X (2014) Transforming growth factor-β1 and α-smooth muscle actin in stromal fibroblasts are associated with a poor prognosis in patients with clinical stage I-IIIA nonsmall cell lung cancer after curative resection. Tumor Biol 35:6707–6713
Ugorski M, Dziegiel P, Suchanski J (2016) Podoplanin - a small glycoprotein with many faces. Am J Cancer Res 6:370–386
Kitano H, Kageyama SI, Hewitt SM, Hayashi R, Doki Y, Ozaki Y, Fujino S, Takikita M, Kubo H, Fukuoka J (2010) Podoplanin expression in cancerous stroma induces lymphangiogenesis and predicts lymphatic spread and patient survival. Arch Pathol Lab Med 134:1520–1527
Neri S, Ishii G, Hashimoto H, Kuwata T, Nagai K, Date H, Ochiai A (2015) Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int J Cancer 137:784–796
Saruwatari K, Ikemura S, Sekihara K, Kuwata T, Fujii S, Umemura S, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Ochiai A, Kohrogi H, Tsuboi M, Goto K, Ishii G (2016) Aggressive tumor microenvironment of solid predominant lung adenocarcinoma subtype harboring with epidermal growth factor receptor mutations. Lung Cancer 91:7–14
Liu AY, Zheng H, Ouyang G (2014) Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol 37:150–156
Soltermann A, Tischler V, Arbogast S, Braun J, Probst-Hensch N, Weder W, Moch H, Kristiansen G (2008) Prognostic significance of epithelial-mesenchymal and mesenchymal- epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res 14:7430–7437
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG (2015) WHO classification of tumours of the lung, pleura, thymus and heart, 4th edn. International Agency for Research on Cancer, Lyon
Liu L, Liu L, Yao HH, Zhu ZQ, Ning ZL, Huang Q (2016) Stromal myofibroblasts are associated with poor prognosis in solid cancers: a meta-analysis of published studies. PLoS One 11:1–16
Kilvaer TK, Khanehkenari MR, Hellevik T et al (2015) Cancer associated fibroblasts in stage I-IIIA NSCLC: prognostic impact and their correlations with tumor molecular markers. PLoS One 10:1–15
Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M (2007) Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res 13:2082–2090
Yamashita M, Ogawa T, Zhang X, Hanamura N, Kashikura Y, Takamura M, Yoneda M, Shiraishi T (2012) Role of stromal myofibroblasts in invasive breast cancer: stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer 19:170–176
Lievense LA, Bezemer K, Aerts JGJV, Hegmans JPJJ (2013) Tumor-associated macrophages in thoracic malignancies. Lung Cancer 80:256–262
Koriyama H, Ishii G, Yoh K, Neri S, Morise M, Umemura S, Matsumoto S, Niho S, Ohmatsu H, Tsuboi M, Goto K, Ochiai A (2015) Presence of podoplanin-positive cancer-associated fibroblasts in surgically resected primary lung adenocarcinoma predicts a shorter progression-free survival period in patients with recurrences who received platinum-based chemotherapy. J Cancer Res Clin Oncol 141:1163–1170
Yoshida T, Ishii G, Goto K, Neri S, Hashimoto H, Yoh K, Niho S, Umemura S, Matsumoto S, Ohmatsu H, Iida S, Niimi A, Nagai K, Ohe Y, Ochiai A (2015) Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin Cancer Res 21:642–651
Kunita A, Baeriswyl V, Meda C, Cabuy E, Takeshita K, Giraudo E, Wicki A, Fukayama M, Christofori G (2018) Inflammatory cytokines induce podoplanin expression at the tumor invasive front. Am J Pathol 188:1276–1288. https://doi.org/10.1016/j.ajpath.2018.01.016
Hong L, Sun H, Lv X, Yang D, Zhang J, Shi Y (2010) Expression of periostin in the serum of NSCLC and its function on proliferation and migration of human lung adenocarcinoma cell line (A549) in vitro. Mol Biol Rep 37:2285–2293
Kikuchi Y, Kunita A, Iwata C, Komura D, Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita Y, Yashiro M, Hirakawa K, Miyazono K, Kudo A, Fukayama M, Kashima TG (2014) The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am J Pathol 184:859–870
Zhao B, Guan H, Liu JQ, Zheng Z, Zhou Q, Zhang J, Su LL, Hu DH (2017) Hypoxia drives the transition of human dermal fibroblasts to a myofibroblast-like phenotype via the TGF-β1/Smad3 pathway. Int J Mol Med 39:153–159
Senavirathna LK, Huang C, Yang X, Munteanu MC, Sathiaseelan R, Xu D et al (2018) Hypoxia induces pulmonary fibroblast proliferation through NFAT signaling. Sci Rep 8:1–16
Madsen CD, Pedersen JT, Venning FA, Singh LB, Moeendarbary E, Charras G et al (2015) Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. EMBO Rep 6:1394–1408
Nishishita R, Morohashi S, Seino H, Wu Y, Yoshizawa T, Haga T, Saito K, Hakamada K, Fukuda S, Kijima H (2018) Expression of cancer-associated fibroblast markers in advanced colorectal cancer. Oncol Lett 15:6195–6202
Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H (2015) Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel) 7:2443–2458
Gascard P, Tlsty TD (2016) Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev 30:1002–1019. https://doi.org/10.1101/gad.279737.116
O’Dwyer DN, Moore BB (2015) The role of periostin in lung fibrosis and airway remodeling. Cell Mol Life Sci 74:4305–4314
Acknowledgments
C.I. is supported by a scholarship from the Takeda Science Foundation (Osaka, Japan).
Author information
Authors and Affiliations
Contributions
C.I. and Y.M. designed the study and wrote the initial draft of the manuscript. C.I. and D.T. performed the experiments and analyzed the data. R.S. evaluated immunohistochemistry and supervised the interpretation of pathology. Y.O. collected tissue samples and supervised the interpretation of clinical data. H.S. supervised all experiments and edited the manuscript. All authors approved the submission of the final manuscript.
Corresponding author
Ethics declarations
The protocol for this study was approved by the Ethics Committee at the Tohoku University School of Medicine (2018-193). This study has been performed in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 128 kb)
Rights and permissions
About this article
Cite this article
Inoue, C., Tamatsuki, D., Miki, Y. et al. Prognostic significance of combining immunohistochemical markers for cancer-associated fibroblasts in lung adenocarcinoma tissue. Virchows Arch 475, 181–189 (2019). https://doi.org/10.1007/s00428-019-02587-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02587-9