Abstract
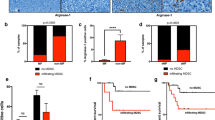
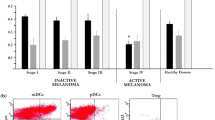
Mycosis fungoides (MF) is characterized by a switch from indolent behaviour in the early stages to a worse clinical outcome in the advanced ones. Recently, various studies have investigated the role the microenvironment might play in such a switch. We have analysed the distribution of Langerhans cells, plasmacytoid dendritic cells and myeloid-derived suppressor cells in 46 MF cases in various stages, aiming to assess whether changes occur from early to advanced stage. We have investigated the number of langerin, CD303 and arginase-1 positive cells and their distribution at high power. Data were analysed using t test for continuous variables, χ 2 tests or Fisher’s exact test for categorical variables, as well as analysis of covariance. In comparing stages IA/B to IIB, we observed a significant decrease in Langerhans cells (p value 0.03) and a significant increase in CD303 and arginase-1 positive cells (p value <0.01 for both markers). Furthermore, a significant increase in Langerhans cells only was observed in stage IIB in comparison to stage III (p = 0.02), while in stage IV, a significant decrease in Langerhans cells was noted in comparison to stage III (p = 0.02). Our data suggest that changes in the microenvironment might influence disease progression, especially from stages IA/B to IIB, opening new scenarios in MF therapy.



Similar content being viewed by others
References
Pileri A Jr, Patrizi A, Agostinelli C et al (2011) Primary cutaneous lymphomas: a reprisal. Semin Diagn Pathol 28:214–233
Quaglino P, Pimpinelli N, Berti E et al (2012) Mycosis fungoides: disease evolution of the “lion queen” revisited. G Ital Dermatol Venereol 147:523–531
Scarisbrick JJ, Prince HM, Vermeer MH et al (2015) Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol 33:3766–3773
Pimpinelli N, Olsen EA, Santucci M et al (2005) Defining early mycosis fungoides. J Am Acad Dermatol 53:1053–1063
Cerroni L (2006) Lymphoproliferative lesions of the skin. J Clin Pathol 59:813–826
Wong HK, Mishra A, Hake T et al (2011) Evolving insights in the pathogenesis and therapy of cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome). Br J Haematol 155:150–166
Wilcox RA (2016) Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 91:151–165
Dunn GP, Old LJ, Schreiber RD (2004a) The three Es of cancer immunoediting. Annu Rev Immunol 22:329–360
Dunn GP, Bruce AT, Ikeda H et al (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991–998
Dunn GP, Old LJ, Schreiber RD (2004b) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21:137–148
Smyth MJ, Godfrey DI, Trapani JA (2001) A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol 2:293–299
Berger CL, Tigelaar R, Cohen J et al (2005) Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood 105:1640–1647
Fried I, Cerroni L (2012) FOXP3 in sequential biopsies of progressive mycosis fungoides. Am J Dermatopathol 34:263–265
Goos M, Kaiserling E, Lennert K (1976) Mycosis fungoides: model for T-lymphocyte homing to the skin? Br J Dermatol 94:221–222
Pimpinelli N, Santucci M, Romagnoli P et al (1994) Dendritic cells in T- and B-cell proliferation in the skin. Dermatol Clin 12:255–270
Lüftl M, Feng A, Licha E et al (2002) Dendritic cells and apoptosis in mycosis fungoides. Br J Dermatol 147:1171–1179
Der-Petrossian M, Valencak J, Jonak C et al (2011) Dermal infiltrates of cutaneous T-cell lymphomas with epidermotropism but not other cutaneous lymphomas are abundant with langerin+ dendritic cells. J Eur Acad Dermatol Venereol 25:922–927
Schlapbach C, Ochsenbein A, Kaelin U et al (2010) High numbers of DC-SIGN+ dendritic cells in lesional skin of cutaneous T-cell lymphoma. J Am Acad Dermatol 62:995–1004
Schwingshackl P, Obermoser G, Nguyen VA et al (2012) Distribution and maturation of skin dendritic cell subsets in two forms of cutaneous T-cell lymphoma: mycosis fungoides and Sézary syndrome. Acta Derm Venereol 92:269–275
Zhang QA, Chen ZQ, Chen MH et al (2014) The number of regular T cells and immature dendritic cells involved in mycosis fungoides is linked to the tumor stage. Eur Rev Med Pharmacol Sci 18:553–558
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 9:162–174
Kumar V, Patel S, Tcyganov E et al (2016) The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 37:208–220
Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40:2969–2975
Bronte V, Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5:641–654
Rodríguez PC, Ochoa AC (2008) Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev 222:180–191
Strauss L, Sangaletti S, Consonni FM et al (2015) RORC1 regulates tumor-promoting “emergency” granulo-monocytopoiesis. Cancer Cell 28:253–269
Rodriguez PC, Hernandez CP, Quiceno D et al (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 202:931–939
Serafini P, Mgebroff S, Noonan K et al (2008) Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 68:5439–5449
Huang B, Pan PY, Li Q et al (2006) Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66:1123–1131
Pan PY, Ma G, Weber KJ et al (2010) Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res 70:99–108
Bunt SK, Yang L, Sinha P et al (2007) Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res 67:10019–10026
Srivastava MK, Sinha P, Clements VK et al (2010) Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 70:68–77
Hanson EM, Clements VK, Sinha P et al (2009) Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol 15(183):937–944
Vardhana S, Younes A (2016) The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica 101:794–802
Wu AA, Drake V, Huang HS et al (2015) Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 4:e1016700
Dette H, Neumeyer N (2001) Nonparametric analysis of covariance. Ann Stat 29:1361–1400
Young SG, Bowman AW (1995) Non-parametric analysis of covariance. Biometrics 51:920–931
Wang XF, Ye D (2010) On nonparametric comparison of images and regression surfaces. J Stat Plann Inference 140:2875–2884
Gabrilovich D, Ishida T, Oyama T et al (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92:4150–4166
Pileri A, Agostinelli C, Righi S et al (2015) Vascular endothelial growth factor A (VEGFA) expression in mycosis fungoides. Histopathology 66:173–181
Kim YH, Gratzinger D, Harrison C et al (2012) In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 119:355–363
Mauti LA, Le Bitoux MA, Baumer K et al (2011) Myeloid-derived suppressor cells are implicated in regulating permissiveness for tumor metastasis during mouse gestation. J Clin Invest 121:2794–2807
Mairhofer DG, Ortner D, Tripp CH et al (2015) Impaired gp100-specific CD8(+) T-cell responses in the presence of myeloid-derived suppressor cells in a spontaneous mouse melanoma model. J Invest Dermatol 135:2785–2793
Pérez C, González-Rincón J, Onaindia A et al (2015) Mutated JAK kinases and deregulated STAT activity are potential therapeutic targets in cutaneous T-cell lymphoma. Haematologica 100:e450–e453
Dufait I, Van Valckenborgh E, Menu E, et al. (2016) Signal transducer and activator of transcription 3 in myeloid-derived suppressor cells: an opportunity for cancer therapy. Oncotarget. [Epub ahead of print].
Fava P, Bergallo M, Astrua C, et al. (2016) miR-155 expression in primary cutaneous T- cell lymphomas (CTCL). J Eur Acad Dermatol Venereol. [Epub ahead of print].
Chen S, Zhang Y, Kuzel TM et al (2015) Regulating tumor myeloid-derived suppressor cells by microRNAs. Cancer Cell Microenviron 2:e637
Kim S, Song JH, Kim S et al (2016) Loss of oncogenic miR-155 in tumor cells promotes tumor growth by enhancing C/EBP-β-mediated MDSC infiltration. Oncotarget 7:11094–11112
Sasso MS, Lollo G, Pitorre M et al (2016) Low dose gemcitabine-loaded lipid nanocapsules target monocytic myeloid-derived suppressor cells and potentiate cancer immunotherapy. Biomaterials 96:47–62
He W, Liang P, Guo G et al (2016) Re-polarizing myeloid-derived suppressor cells (MDSCs) with cationic polymers for cancer immunotherapy. Sci Rep 6:24506
Campbell JJ, Clark RA, Watanabe R et al (2010) Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood 116:767–771
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the patients provided their consent to the study, which was approved by the local Review Board and was conducted in accordance with the Helsinki Declaration.
Funding sources
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Pileri, A., Agostinelli, C., Sessa, M. et al. Langerhans, plasmacytoid dendritic and myeloid-derived suppressor cell levels in mycosis fungoides vary according to the stage of the disease. Virchows Arch 470, 575–582 (2017). https://doi.org/10.1007/s00428-017-2107-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2107-1