Abstract
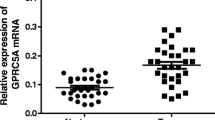
Gastric cancer (GC) is one of the most common causes of cancer-related deaths worldwide. We investigated the differential expression and putative tumor biological significance of five G-protein-coupled receptors (GPCRs) in GC, i.e., LGR4, LGR6, GPR34, GPR160, and GPR171. Based on our previous microarray analyses, we identified five candidate genes in human GC samples. Real-time RT-PCR was carried out to validate their expression in malignant and non-malignant tissues on an independent collective comprising 32 GC patients with and without lymph node metastases. Selected protein targets LGR4 and LGR6 were further validated on paraffin-embedded sections of ten intestinal and ten poorly cohesive (diffuse)-type GCs and their corresponding non-malignant tissue using immunohistochemistry. Additionally, the putative tumor biological significance of LGR4 and LGR6 was studied using tissue microarrays obtained from a cohort of 481 GC patients. On transcriptional level, GPR34, GPR160, and GPR171 were not differentially expressed in GC compared with non-neoplastic mucosa. LGR4 and LGR6 were up-regulated on transcriptional (real-time RT-PCR) and translational (immunohistochemistry) levels in GC. Furthermore, in tissue microarray analysis, LGR6 expression was significantly associated with local tumor growth (T-category; p = 0.04) and correlated with patient survival. LGR4 expression was significantly correlated with nodal spread (N-category; p = 0.025). Our systematic analysis indicates that LGR4 and LGR6 may play a role in GC biology. Future studies will have to demonstrate whether these are also putative diagnostic, prognostic, and/or therapeutic targets for GC.




Similar content being viewed by others
References
Krejs GJ (2010) Gastric cancer: epidemiology and risk factors. Dig Dis 28:600–603
Garlipp B, Schwalenberg J, Adolf D, Lippert H, Meyer F (2011) Epidemiology, surgical management and early postoperative outcome in a cohort of gastric cancer patients of a tertiary referral center in relation to multi-center quality assurance studies. Pol Przegl Chir 83:123–134
Crew KD, Neugut AI (2006) Epidemiology of gastric cancer. World J Gastroenterol 12:354–362
Correa P, Piazuelo MB, Camargo MC (2006) Etiopathogenesis of gastric cancer. Scand J Surg 95:218–224
Moehler M, Al-Batran SE, Andus T et al und AWMF (2011) German S3-guideline “Diagnosis and treatment of esophagogastric cancer”. Z Gastroenterol 49:461–531
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK und Investigators, ToGA Trial (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Böhme I, Beck-Sickinger AG (2009) Illuminating the life of GPCRs. Cell Commun Signal 7:16
Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650
Ingold B, Simon E, Ungethüm U, Kuban RJ, Müller BM, Lupp A, Neumann U, Ebert MP, Denkert C, Weichert W, Schulz S, Röcken C (2010) Vascular CXCR4 expression—a novel antiangiogenic target in gastric cancer? PLoS One 5:e10087
Schmuck R, Warneke V, Behrens HM, Simon E, Weichert W, Röcken C (2011) Genotypic and phenotypic characterization of side population of gastric cancer cell lines. Am J Pathol 178:1792–1804
Leushacke M, Barker N (2011) LGR5 and LGR6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene. doi:10.1038/onc.2011.479
Barker N, Clevers H (2010) Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138:1681–1696
Simon E, Petke D, Böger C, Behrens HM, Warneke V, Ebert M, Röcken C (2012) The spatial distribution of LGR5(+) cells correlates with gastric cancer progression. PLoS One 7:e35486
Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ (2000) The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol 14:1257–1271
de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H (2011) LGR5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476:293–297
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumors of the digestive system, 4th edn. International Agency for Research on Cancer, Lyon
Sobin LH, Gospodarowicz MK, Wittekind C (2010) International Union Against Cancer (UICC) TNM classification of malignant tumors, 7th edn. Wiley-Liss, New York
Bornschein J, Kandulski A, Selgrad M, Malfertheiner P (2010) From gastric inflammation to gastric cancer. Dig Dis 28:609–614
Carl-McGrath S, Ebert M, Röcken C (2007) Gastric adenocarcinoma: epidemiology, pathology and pathogenesis. Cancer therapy 5:877–894
Mazerbourg S, Bouley DM, Sudo S, Klein CA, Zhang JV, Kawamura K, Goodrich LV, Rayburn H, Tessier-Lavigne M, Hsueh AJ (2004) Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol Endocrinol 18:2241–2254
Birchmeier W (2011) Stem cells: orphan receptors find a home. Nature 476:287–288
Carmon KS, Gong X, Lin Q, Thomas A, Liu Q (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 108:11452–11457
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene LGR5. Nature 449:1003–1007
Gao Y, Kitagawa K, Hiramatsu Y, Kikuchi H, Isobe T, Shimada M, Uchida C, Hattori T, Oda T, Nakayama K, Nakayama KI, Tanaka T, Konno H, Kitagawa M (2006) Up-regulation of GPR48 induced by down-regulation of p27Kip1 enhances carcinoma cell invasiveness and metastasis. Cancer Res 66:11623–11631
Gao Y, Shan ZY, Wang H, Zhang HM, Teng WP (2009) Inhibitory effect of shRNA targeting GPR48 on invasion and metastasis of human cervical carcinoma cell line HeLa. Ai Zheng 28:104–107
Mustata RC, Van Loy T, Lefort A, Libert F, Strollo S, Vassart G, Garcia MI (2011) LGR4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep 12:558–564
Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D (2006) G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol 84:287–297
Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgård R, Clevers H (2010) LGR6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327:1385–1389
Acknowledgments
We would like to thank Sandra Krüger for her excellent technical support. This study was supported by grants from Deutsche Forschungsgemeinschaft (Ro 1173/12).
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosures
There are no potential conflicts (financial, professional, or personal) to disclose that are relevant to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Assessment of LGR4/LGR6-co-expression in tissue micro arrays and correlation with clinico-pathological patient characteristics. P-values were calculated with Fisher's exact test (PDF 165 kb)
Online Resource 2
Patient survival of the cases co-expressing LGR4 and LGR6 compared to those negative for both markers. Results are presented as Kaplan–Meier-curves. The p-value was calculated using the log-rank (Mantel–Cox) test (JPEG 40 kb)
Rights and permissions
About this article
Cite this article
Steffen, J.S., Simon, E., Warneke, V. et al. LGR4 and LGR6 are differentially expressed and of putative tumor biological significance in gastric carcinoma. Virchows Arch 461, 355–365 (2012). https://doi.org/10.1007/s00428-012-1292-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-012-1292-1