Abstract
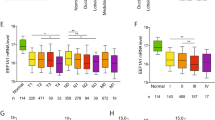
Proline-, glutamic acid-, and leukine-rich protein (PELP1) is a novel co-regulatory protein that modulates genomic and non genomic actions of estrogen receptors. Nuclear receptor co-repressor (NCoR) represses estrogen-receptor-dependent transcription. PELP1 and NCoR expression was evaluated in tissue sections from 107 formalin-fixed, paraffin-embedded colectomy specimens. Normal mucosa and adenomas were also evaluated in 77 and 29 cases, respectively. PELP1 was expressed in a dot-like pattern in the nuclei of epithelial and stromal cells. Statistical analysis revealed an increase in PELP1 expression in myofibroblasts from normal mucosa through adenomas to carcinomas. NCoR was expressed in the nuclei and the cytoplasm of epithelial cells. Nuclear expression was more common in normal mucosa, whereas cytoplasmic expression was higher in malignant epithelial cells. Additionally, NCoR was expressed in the cytoplasm of cancer-associated myofibroblasts, but was rarely noted in myofibroblasts of normal mucosa or adenomas. Cytoplasmic expression of NCoR in epithelial cells correlated with better disease-free and overall survival on univariate analysis and was an independent prognostic marker for disease-free survival on multivariate analysis. These findings suggest that deregulation of co-regulators expression in both epithelial cells and myofibroblasts may contribute to the initiation and progression of colorectal carcinoma.



Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002 CA. Cancer J Clin 55:74–108
Kampman E, Potter JD, Slattery ML et al (1997) Hormone replacement therapy, reproductive history, and colon cancer: a multicenter, case-control study in the United States. Cancer Causes Control 8:146–158
Lechner D, Kallay E, Cross HS (2005) Phytoestrogens and colorectal cancer prevention. Vitam Horm 70:169–198
Gunter MJ, Hoover DR, Yu H et al (2008) Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res 68:329–337
Di Domenico M, Castoria G, Bilancio A et al (1996) Estradiol activation of human colon carcinoma-derived Caco-2 cell growth. Cancer Res 56:4516–4521
Deroo B, Korach KS (2006) Estrogen receptors and human disease. J Clin Invest 116:561–570
Lonard DM, O’Malley BW (2007) Nuclear receptor coregulators: judges, juries and executioners of cellular regulation. Mol Cell 27:691–700
McKenna NJ, O’Malley BW (2002) Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474
O’Malley BW (2006) Little molecules with big goals. Science 313:1749–1750
McKenna NJ, Lanz RB, O’Malley BW (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344
Wong C-W, McNally C, Nickbarg E et al (2002) Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src Erk phosphorylation cascade. Proc Natl Acad Sci USA 99:14783–14788
Wu RC, Qin J, Yi P et al (2004) Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic responses to multiple cellular signalling pathways. Mol Cell 15:937–949
Greger JG, Fursov N, Cooch N (2007) Phosphorylation of MNAR promotes estrogen activation of phosphatidylinositol 3-kinase. Mol Cel Biol 27:1904–1913
Smith CL, O’Malley BW (2004) Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71
Webb P, Valentine C, Nguyen P et al (2003) ERβ binds N-CoR in the presence of estrogens via an LXXLL-like motif in the N-CoR C-terminus. Nucl Recept 1:4
Fernadez-Majada V, Pujadas J, Vilardell F et al (2007) Aberrant cytoplasmic localization of N-CoR in colorectal tumors. Cell cycle 6:1748–1752
Espinosa L, Santos S, Ingles-Esteve J et al (2002) p65-NFkappaB synergizes with Notch to activate transcription by triggering cytoplasmic translocation of the nuclear receptor corepressor N-CoR. J Cell Sci 115:1295–1303
Jonas BA, Privalsky ML (2004) SMRT and N-CoR corepressors are regulated by distinct kinase signalling pathways. J Biol Chem 279:54676–54686
Rajhans R, Vadlamudi RK (2006) Comprehensive analysis of recent biochemical and biologic findings regarding a newly discovered protein-PELP1/MNAR. Clin Exp Metastasis 23:1–7
Vadlamudi RK, Wang RA, Mazumdar A et al (2001) Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor α. J Biol Chem 276:38272–38279
Witz IP (2008) Yin–yang activities and vicious cycles in the tumor microenvironment. Cancer Res 68:9–13
Chang HY, Sneddon JB, Alizadeh AA et al (2004) Gene expression signature of fibroblast serum response predicts cancer progression: similarities between tumors and wounds. PLOS Biol 2:e7
West RB, Nuyten DSA, Subramanian S et al (2005) Determination of stroma signatures in breast carcinoma. PLOS Biol 3:e187
Desmoulliere A, Guyot C, Gabbiani G (2004) The stroma reaction myofibroblast: key player in the control of tumor cell behavior. Int J Dev Biol 48:509–517
Nakayama H, Enzan H, Miyazaki E et al (2000) Differential expression of CD34 in normal colorectal tissue, peritumoral inflammatory tissue, and tumor stroma. J Clin Pathol 53:626–629
Adegboyega PA, Mifflin RC, DiMari JF et al (2002) Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps and adenomatous colorectal polyps. Arch Pathol Lab Med 126:829–836
Chen AL, Soman KV, Rychahou PG et al (2005) Proteomic analysis of colonic myofibroblasts and effect on colon cancer cell proliferation. Surgery 138:382–390
De Wever O, Nguyen OD, Van Hoorde L et al (2004) Tenascin C and SF/HGF produced by myofibroblasts can provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J 18:1016–1028
Sivridis E, Giatromanolaki A, Koukourakis MI (2005) Proliferating fibroblasts at the invading tumour edge of colorectal adenocarcinomas are associated with endogenous markers of hypoxia, acidity, and oxidative stress. J Clin Pathol 58:1033–1038
Liebeau B, Heymann M-F, Henry F et al (1999) Immunomodulatory effects of tumor associated fibroblasts in colorectal tumor development. Int J Cancer 81:629–636
Scoville DH, Sato T, He XC et al (2008) Current view: intestinal stem cells and signaling. Gastroenterology 134:849–864
Powell DW, Mifflin RC, Valentich JD et al (1999) Mofibroblast I. Paracrine cells important in health and disease. Am J Physiol 277(1 Pt 1):C1–C9
Cooke PS, Buchanan DL, Young P et al (1997) Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA 94:6535–6540
Cunha GR, Young P, Hom YK et al (1997) Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J Mammary Gland Biol Neoplasia 2:393–402
Prins GS, Birch L, Couse JF et al (2001) Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res 61:6089–6097
Gupta PB, Proia D, Cingoz O et al (2007) Systemic stromal effects of estrogens promote the growth of estrogen receptor-negative tumors. Cancer Res 67:2062–2071
Schurch W, Seemayer TA, Gabbiani G (1998) The myofibroblast. Am J Surg Pathol 22:141–147
Frasor J, Danes JM, Funk CC et al (2005) Estrogen down-regulation of the corepressor N-CoR: mechanism and implications for estrogen derepression of N-CoR-regulated genes. PNAS 102:13153–13157
Dobrzycka KM, Townson SM, Jiang S et al (2003) Estrogen receptor corepressors—a role in human breast cancer. Endocr Relat Cancer 10:517–536
Park DM, Li J, Okamoto H, Akeju O et al (2007) N-CoR pathway targeting induces glioblastoma derived cancer stem cell differentiation. Cell Cycle 6:467–470
Lee M-O, Kang H-J (2002) Role of coactivators and corepressors in the induction of the RARβ gene in human colon cancer cells. Biol Pharm Bull 25:1298–1302
Hornberg JJ, Bruggeman FJ, Westerhoff HV et al (2006) Cancer: a system biology disease. Biosystems 83:81–90
Jepsen K, Hermanson OM, Onami TM et al (2000) Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102:753–763
Hermanson O, Jepsen K, Rosenfeld MG (2002) N-CoR controls differentiation of neural stem cells into astrocytes. Nature 419:934–939
Grignani F, De Matteis S, Nervi C et al (1998) Fusion proteins of the retinoic acid receptor-a recruit histone deacetylase in promyelocytic leukaemia. Nature 391:815–818
Fortunel NO, Otu HH, Ng HH et al (2000) Comment on “‘stemness’: transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature”. Science 302:393
Spirakov M, Fisher AG (2007) Epigenetic signatures of stem-cell identity. Nat Rev Genet 8:263–271
Vadlamudi RK, Kumar R (2007) Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nuclear Receptor Signaling 5:e004
Barleta F, Wong C-W, McNally C et al (2004) Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol 18:1096–1108
Vadlamudi RK, Manavathi B, Balasenthil S et al (2005) Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res 65:7724–7732
Nair SS, Mishra SK, Yang Z et al (2004) Potential role of a novel transcriptional coactivators PELP1 in histone H1 displacement in cancer cells. Cancer Res 64:6416–6423
King KJ, Nicholson HD, Assinder SJ (2006) Effect of increasing ratio of estrogen:androgen on proliferation of normal human prostate stromal and epithelial cells, and the malignant cell line LNCaP. Prostate 66:105–114
Rajhans R, Nair S, Holden AH et al (2007) Oncogenic potential of the nuclear receptor coregulator proline-, glutamic acid-, leucine-rich protein 1/modulator of the nongenomic actions of the estrogen receptor. Cancer Res 67:5505–5512
Nair S, Vadlamudi RK (2007) Emerging significance of ER-coregulator PELP1/MNAR in cancer. Histol Histopathol 22:91–96
Horard B, Vanacker J-M (2003) Estrogen receptor-related receptors: orphan receptors desperately seeking a ligand. J Mol Endocrinol 31:349–357
Cavallini A, Notarnicola M, Giannini P et al (2005) Oestrogen receptor-related receptor alpha (ERRa) and oestrogen receptors (ERa and ERb) exhibit different gene expression in human colorectal tumour progression. Eur J Cancer 41:1487–1494
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00428-008-0719-1
Rights and permissions
About this article
Cite this article
Tzelepi, V., Grivas, P., Kefalopoulou, Z. et al. Expression of estrogen receptor co-regulators NCoR and PELP1 in epithelial cells and myofibroblasts of colorectal carcinomas: cytoplasmic translocation of NCoR in epithelial cells correlates with worse prognosis. Virchows Arch 454, 41–53 (2009). https://doi.org/10.1007/s00428-008-0708-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-008-0708-4