Abstract
Main conclusions
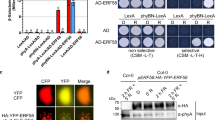
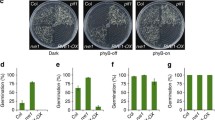
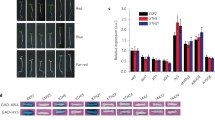
During the light induction of somatic embryogenesis, phyB-Pfr suppresses Phytoglobin 2, known to elevate nitric oxide (NO). NO depresses Phytochrome Interacting Factor 4 (PIF4) relieving its inhibition on embryogenesis through auxin.
Abstract
An obligatory step of many in vitro embryogenic systems is the somatic–embryogenic transition culminating with the formation of the embryogenic tissue. In Arabidopsis, this transition requires light and is facilitated by high levels of nitric oxide (NO) generated by either suppression of the NO scavenger Phytoglobin 2 (Pgb2), or its removal from the nucleus. Using a previously characterized induction system regulating the cellular localization of Pgb2, we demonstrated the interplay between phytochrome B (phyB) and Pgb2 during the formation of embryogenic tissue. The deactivation of phyB in the dark coincides with the induction of Pgb2 known to reduce the level of NO; consequently, embryogenesis is inhibited. Under light conditions, the active form of phyB depresses the levels of Pgb2 transcripts, thus expecting an increase in cellular NO. Induction of Pgb2 increases Phytochrome Interacting Factor 4 (PIF4) suggesting that high levels of NO repress PIF4. The PIF4 inhibition is sufficient to induce several auxin biosynthetic (CYP79B2, AMI1, and YUCCA 1, 2, and 6) and response (ARF5, 8, and 16) genes, conducive to the formation of the embryonic tissue and production of somatic embryos. Auxin responses mediated by ARF10 and 17 appear to be regulated by Pgb2, possibly through NO, in a PIF4-independent fashion. Overall, this work provides a new and preliminary model integrating Pgb2 (and NO) with phyB in the light regulation of in vitro embryogenesis.






Similar content being viewed by others
Data availability
All materials described in the manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Abbreviations
- AMI1:
-
Amidase 1
- ARF:
-
Auxin response factor
- NO:
-
Nitric oxide
- phyB:
-
Phytochrome B
- Pgb:
-
Phytoglobin
- PIF:
-
Phytochrome interacting factor
- SE:
-
Somatic embryogenesis
References
Bai B, Su YH, Yuan J, Zhang XS (2013) Induction of somatic embryos in Arabidopsis requires local YUCCA expression mediated by the down-regulation of ethylene biosynthesis. Mol Plant 6:1247–1260
Bai S, Yao T, Li M, Guo X, Zhang Y, Zhu S, He Y (2014) PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol Plant 7:616–625
Bassuner BMR, Lam R, Lukowitz W, Yeung EC (2007) Auxin and root initiation in somatic embryos of Arabidopsis. Plant Cell Rep 26:1–11
Beligni MV, Lamattina L (2000) Nitric oxide induces seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210:215–221
Castillon A, Shen L, Huq B (2007) Phytochrome interacting factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci 12:514–521
Chan A, Stasolla C (2023) Light induction of somatic embryogenesis in Arabidopsis is regulated by PHYTOCHROME E. Plant Physiol Biochem 195:163–169
Chen M, Chory J (2011) Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol 21:664–667
Chen JR, Wu L, Hu BW, Yi X, Liu R, Deng ZN, Xiong XY (2014) The influence of plant growth regulators and light quality on somatic embryogenesis in China Rose (Rosa chinensis Jacq.). J Plant Growth Regul 33:295–304
Chen CC, Agrawal DC, Lee MR, Lee RJ, Kuo CL, Wu CR, Tsay HS, Chang HC (2016) Influence of LED light spectra on in vitro somatic embryogenesis and LC–MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb.: a medicinal herb. Bot Stud 57:9–12
Christie JM, Blackwood L, Petersen J, Sullivan S (2015) Plant flavoprotein photoreceptors. Plant Cell Physiol 56:401–413
D’Onofrio C, Morini S, Bellocchi G (1998) Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tiss Organ Cult 53:91–98
Elhiti M, Hebelstrup KH, Wang A, Li C, Cui Y, Hill RD, Stasolla C (2013) Function of type-2 Arabidopsis hemoglobin in the auxin-mediated formation of embryogenic cells during morphogenesis. Plant J 74:946–958
Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Ye S, Yu P, Breen G, Cohen J (2011) Phytochrome interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci USA 108:20231–20235
Gaj MD (2002) Stimulation of somatic embryo formation by mutagens and darkness in culture of immature zygotic embryos of Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 37:93–98
Geuens E, Brouns I, Flamez D, Dewilde S, Timmermans JP, Moens L (2003) A globin in the nucleus! J Biol Chem 278:30417–30420
Godee C, Mira MM, Wally O, Hill RD, Stasolla C (2017) Cellular localization of the Arabidopsis class 2 phytoglobin influences somatic embryogenesis. J Exp Bot 68:1013–1023
Hebelstrup KH, Jensen EO (2008) Expression of NO scavenging hemoglobin is involved in the timing of bolting in Arabidopsis thaliana. Planta 227:917–927
Hill RD (2012) Non-symbiotic haemoglobins - What’s happening beyond nitric oxide scavenging? AoB Plants. https://doi.org/10.1093/aobpla/pls004
Huang S, Hill RD, Wally OS, Dionisio G, Ayele BT, Jami SK, Stasolla C (2014a) Hemoglobin control of cell survival/death decision regulates in vitro plant embryogenesis. Plant Physiol 23:456–464
Huang S, Hill R, Stasolla C (2014b) Plant hemoglobin participation in cell fate determination. Plant Signal Behav. https://doi.org/10.4161/psb.29485
Hundahl CA, Allen GC, Hannibal J, Kjaer K, Rehfeld JF, Dewilde S, Nyengaard JR, Kelsen J, Hay-Schmidt A (2010) Anatomical characterization of cytoglobin and neuroglobin mRNA and protein expression in the mouse brain. Brain Res 1331:58–67
Huq E, Quail PH (2002) PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J 21:2441–2450
Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55:2473–2482
Jang IC, Yang JY, Seo HS, Chua NH (2005) HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome a signaling. Genes Dev 19:593–602
Jeong J, Choi G (2013) Phytochrome-interacting factors have both shared and distinct biological roles. Mol Cells 35:371–380
Khanna R, Huq E, Kikis EA, Al-Sady B, Lanzatella C, Quail PH (2004) A Novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 16:3033–3044
Li J, Li G, Wang H, Wang Deng X (2011) Phytochrome signaling mechanisms. Arabidopsis Book. https://doi.org/10.1199/tab.0148
Li R, Jia Y, Yu L, Yang W, Chen Z, Chen H, Hu X (2018) Nitric oxide promotes light-initiated seed germination by repressing PIF1 expression and stabilizing HFR1. Plant Physiol Biochem 123:204–212
Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yang HQ (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20:292–306
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Lopes-Oliveira PJ, Oliveira HC, Kolbert Z, Freschi L (2021) The light and dark sides of nitric oxide: multifaceted roles of nitric oxide in plant responses to light. J Exp Bot 72:885–903
Lorrain S, Trevisan M, Pradervand S, Fankhauser C (2009) Phytochrome interacting factors 4 and 5 redundantly limit seedling de-etiolation in continuous far-red light. Plant J 60:449–461
Lozano-Juste J, León J (2011) Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis. Plant Physiol 156:1410–1423
Ma D, Li X, Guo Y, Chu J, Fang S, Yan C, Liu H (2016) Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc Natl Acad Sci USA 113:224–229
Mancinelli A (1994) The physiology of phytochrome action. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in plants. Kluwer Academic Publishers, Dordrecht, pp 211–269
Mashiguchi K, Tanaka K, Sakai T (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Nat Acad Sci USA 108:18512–18517
Melo NK, Bianchetti RE, Lira BS, Oliveira PM, Zuccarelli R, Dias DL, Demarco D, Peres LE, Rossi M, Freschi L (2016) Nitric oxide, ethylene, and auxin cross talk mediates greening and plastid development in de-etiolating tomato seedlings. Plant Physiol 170:2278–2294
Mendez-Hernandez HA, Ledezma-Rodriguez MA, Avilez-Montalvo RN, Juarez-Gomez YL, Skeete A, Avilez-Montalvo J, de-La-Pena C, Loyola-Vargas VM, (2019) Signaling overview of plant somatic embryogenesis. Front Plant Sci 10:77. https://doi.org/10.3389/fpls.2019.00077
Micheli M, Standardi A (2016) From somatic embryo to synthetic seed in citrus spp. through the encapsulation technology. Methods Mol Biol 1359:515–522
Michler CH, Lineberger RD (1987) Effects of light on somatic embryo development and abscisic levels in carrot suspension cultures. Plant Cell Tiss Organ Cult 11:189–207
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Otvos K, Pasternak TP, Miskolczi P, Domoki M, Dorjgotov D, Szcs A, Bottka S, Dudits D, Feher A (2005) Nitric oxide is required for, and promotes auxin-mediated activation of, cell division and embryogenic cell formation but does not influence cell cycle progression in alfalfa cell cultures. Plant J 43:849–860
Pais MS (2019) Somatic embryogenesis induction in woody species: the future after OMICs data assessment. Front Plant Sci 10:240–257
Pham VN, Xu X, Huq E (2018) Molecular bases for the constitutive photomorphogenic phenotypes in Arabidopsis. Development 145:145–153
Quail PH (2002) Phytochrome photosensory signalling networks. Mol Cell Biol 3:85–93
Reinert J (1958) Morphogenese und ihre kontrolle an gewebekulturen aus carotten. Naturwissenschaften 45:344–345
Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, Ulm R (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332:103–106
Rockel P, Strube F, Rockel A, Wildt J, Kaiser W (2022) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110
Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW (2003) The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev 17:2642–2647
Senger H, Schmidt W (1994) Diversity of photoreceptors. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in plants. Kluwer Academic Publishers, Dordrecht, pp 301–325
Seo HS, Yang JY, Ishikawa M, Bolle C, Ballesteros ML, Chua NH (2003) LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423:995–999
Seregélyes C, Mustárdy L, Ayaydin F (2000) Nuclear localization of a hypoxia-inducible novel non-symbiotic hemoglobin in cultured alfalfa cells. FEBS Lett 482:125–130
Sookyung O, Beronda L, Montgomery B (2014) Phytochrome-dependent coordinate control of distinct aspects of nuclear and plastid gene expression during anterograde signaling and photomorphogenesis. Front Plant Sci 5:171. https://doi.org/10.3389/fpls.2014.00171
Stasolla C, Huang S, Hill RD, Igamberdiev AU (2019) Spatio-temporal expression of phytoglobin: a determining factor in the NO specification of cell fate. J Exp Bot 70:4365–4377
Su YH, Zhang XS (2009) Auxin gradients trigger de novo formation of stem cells during somatic embryogenesis. Plant Signal Behav 4:574–576
Sun J, Qi L, Li Y, Chu J, Li C (2012) PIF4–mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet 8(3):e1002594
Sun J, Qi L, Li Y, Zhai Q, Li C (2013) PIF4 and PIF5 transcription factors link blue light and auxin to regulate the phototropic response in Arabidopsis. Plant Cell 25:2102–2114
Wójcikowska B, Gaj MD (2016) Somatic embryogenesis in Arabidopsis. In: Loyola-Vargas V, Ochoa-Alejo N (eds) Somatic embryogenesis: fundamental aspects and applications. Springer, Cham, Switzerland, pp 185–199
Wójcikowska B, Gaj, (2017) Expression profiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep 36:843–858
Xu Y, Zhu Z (2021) PIF4 and PIF4-interacting proteins: at the nexus of plant light, temperature and hormone signal integrations. Int J Mol Sci 24:22–26
Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A (1999) Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol 145:437–445
Yoshida Y, Sarmiento-Mañús R, Yamori W, Ponce MR, Micol JL, Tsukaya H (2018) The Arabidopsis phyB-9 mutant has a second-site mutation in the VENOSA4 gene that alters chloroplast size, photosynthetic traits, and leaf growth. Plant Physiol 178:3–6
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Acknowledgements
This work was supported by a NSERC Discovery Grant (126542) to CS.
Funding
NSERC, 13245, Claudio Stasolla.
Author information
Authors and Affiliations
Contributions
SD performed the somatic embryogenesis experiments with different light conditions. MM performed the remaining experiments and contributed to the writing of the ms. RDH contributed with the conceptualization of the experiments and interpretation of data. CC conceptualized the work, contributed with the interpretation of data and helped writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mira, M.M., Day, S., Ibrahim, S. et al. The Arabidopsis Phytoglobin 2 mediates phytochrome B (phyB) light signaling responses during somatic embryogenesis. Planta 257, 88 (2023). https://doi.org/10.1007/s00425-023-04121-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04121-3