Abstract
Main conclusion
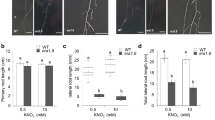
The peptidyl-prolyl isomerases FKBP15-1 and FKBP15-2 negatively modulate lateral root development by repressing vacuolar invertase VIN2 activity.
Abstract
Lateral root (LR) architecture greatly affects the efficiency of nutrient absorption and the anchorage of plants. Although the internal phytohormone regulatory mechanisms that control LR development are well known, how external nutrients influence lateral root development remains elusive. Here, we characterized the function of two FK506-binding proteins, namely, FKBP15-1 and FKBP15-2, in Arabidopsis. FKBP15-1/15–2 genes were expressed prominently in the vascular bundles of the root basal meristem region, and the FKBP15-1/15–2 proteins were localized to the endoplasmic reticulum of the cells. Using IP-MS, Co-IP, and BiFC assays, we demonstrated that FKBP15-1 and FKBP15-2 interacted with vacuolar invertase 2 (VIN2). Compared to Col-0 and the single mutants, the fkbp15-1fkbp15-2 double mutant had more LRs, and presented higher sucrose catalytic activity. Moreover, genetic analysis showed genetic epistasis of VIN2 over FKBP15-1/FKBP15-2 in controlling LR development. Our results indicate that FKBP15-1 and FKBP15-2 participate in the control of LR number by inhibiting the catalytic activity of VIN2. Owing to the conserved peptidylprolyl cis–trans isomerase activity of FKBP family proteins, our results provide a clue for further analysis of the interplay between lateral root development and protein modification by FKBPs.







Similar content being viewed by others
Abbreviations
- LR:
-
Lateral root
- FKBP:
-
FK506-binding proteins
- IP-MS:
-
Immunoprecipitation and mass spectrometry
- VIN2:
-
Vacuolar invertase 2
- XPP:
-
Xylem pole-pericycle
- ER:
-
Endoplasmic reticulum
- UBQ:
-
Ubiquitin
- GUS:
-
ß-glucuronidase
- YFP:
-
Yellow fluorescent protein
- GFP:
-
Green fluorescent protein
- MS:
-
Murashige and Skoog
- TOR:
-
Target of rapamycin
- TWD1:
-
TWISTED DWARF1
References
Alonso JM, Stepanova AN, Leisse TJ et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Bai H, Murali B, Barber K et al (2013) Low phosphate alters lateral root setpoint angle and gravitropism. Am J Bot 100:175–182
Benitez-Alfonso Y, Faulkner C, Pendle A et al (2013) Symplastic intercellular connectivity regulates lateral root patterning. Dev Cell 26:136–147
Benková E, Bielach A (2010) Lateral root organogenesis — from cell to organ. Curr Opin Plant Biol 13:677–683
Bouchard R, Bailly A, Blakeslee JJ et al (2006) Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. J Biol Chem 281:30603–30612
Brady SM, Orlando DA, Lee JY et al (2007) A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318:801–806
Brauer EK, Ahsan N, Dale R et al (2016) The Raf-like kinase ILK1 and the high affinity K+ transporter HAK5 are required for innate immunity and abiotic stress response. Plant Physiol 171:1470–1484
Casimiro I, Beeckman T, Graham N et al (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8:165–171
Chapman K, Taleski M, Ogilvie HA, Imin N, Djordjevic MA (2019) CEP-CEPR1 signalling inhibits the sucrose-dependent enhancement of lateral root growth. J Exp Bot 70:3955–3967
Chen BB, Wang J, Zhang J et al (2016) Two types of soybean diacylglycerol acyltransferases are differentially involved in triacylglycerol biosynthesis and response to environmental stresses and hormones. Sci Rep 6:28541
Cho H, Ryu H, Rho S et al (2014) A secreted peptide acts on BIN2-mediated phosphorylation of ARFs to potentiate auxin response during lateral root development. Nat Cell Biol 16:66–76
De Smet I (2012) Lateral root initiation: one step at a time. New Phytol 193:867–873
Du Y, Scheres B (2018) Lateral root formation and the multiple roles of auxin. J Exp Bot 69:155–167
Duque LO, Villordon A (2019) Root branching and nutrient efficiency: status and way forward in root and tuber crops. Front Plant Sci 10:237
Durand M, Mainson D, Porcheron B et al (2018) Carbon source-sink relationship in Arabidopsis thaliana: the role of sucrose transporters. Planta 247:587–611
Fukaki H, Tasaka M (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69:437–449
Galat A (2003) Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity-targets-functions. Curr Top Med Chem 3:1315–1347
Geisler M, Girin M, Brandt S et al (2004) Arabidopsis immunophilin-like TWD1 functionally interacts with vacuolar ABC transporters. Mol Biol Cell 15:3393–3405
Gupta A, Singh M, Mishra BS et al (2009) Role of glucose in spatial distribution of auxin regulated genes. Plant Signal Behav 4:862–863
Gupta A, Singh M, Laxmi A (2015) Interaction between glucose and brassinosteroid during the regulation of lateral root development in Arabidopsis. Plant Physiol 168:307–320
Hammond JP, White PJ (2011) Sugar signaling in root responses to low phosphorus availability. Plant Physiol 156:1033–1040
Harikishore A, Yoon HS (2015) Immunophilins: structures, mechanisms and ligands. Curr Mol Pharmacol 9:37–47
Helliwell C, Waterhouse P (2003) Constructs and methods for high-throughput gene silencing in plants. Methods 30:289–295
Hooper CM, Castleden IR, Tanz SK et al (2017) SUBA4: the interactive data analysis centre for Arabidopsis subcellular protein locations. Nucleic Acids Res 45:D1064–D1074
Ivanchenko MG, Zhu J, Wang B et al (2015) The cyclophilin A DIAGEOTROPICA gene affects auxin transport in both root and shoot to control lateral root formation. Development 142:712–721
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jeon J, Cho C, Lee MR et al (2016) CYTOKININ RESPONSE FACTOR2 (CRF2) and CRF3 regulate lateral root development in response to cold stress in Arabidopsis. Plant Cell 28:1828–1843
Jing HW, Strader LC (2019) Interplay of auxin and cytokinin in lateral root development. Int J Mol Med Sci 20:486
Jing H, Strader CL (2019) Interplay of auxin and cytokinin in lateral root development. Int J Mol Sci 20:486
Lavenus J, Lucas M, Laplaze L et al (2013) The dicot root as a model system for studying organogenesis. Methods Mol Biol 959:45–67
Leskow CC, Kamenetzky L, Dominguez PG et al (2016) Allelic differences in a vaculoar invertase affect Arabidopsis growth at early plant development. J Exp Bot 67(14):4091–4103
Lima A, Lima S, Wong JH et al (2006) A redox-active FKBP-type immunophilin functions in accumulation of the photosystem II supercomplex in Arabidopsis thaliana. Proc Natl Acad Sci U S A 103:12631–12636
Liu W, Han X, Zhan G et al (2015) A novel sucrose-regulatory MADS-Box transcription factor GmNMHC5 promotes root development and nodulation in soybean (Glycine max [L.] Merr.). Int J Mol Sci 16:20657–20673
Ljung K, Nemhauser JL, Perata P (2015) New mechanistic links between sugar and hormone signalling networks. Curr Opin Plant Bio 25:130–137
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127:899–909
McCleery WT, Mohd-Radzman NA, Grieneisen VA (2017) Root branching plasticity: collective decision-making results from local and global signalling. Curr Opin Cell Biol 44:51–58
Mishra BS, Singh M, Aggrawal P et al (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 4:e4502
Mogami J, Fujita Y, Yoshida T et al (2014) Two distinct families of protein kinases are required for plant growth under high external Mg2+ concentrations in Arabidopsis. Plant Physiol 55:781–789
Morenorisueno MA, Norman JMV, Moreno A et al (2010) Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329:1306–1311
Morey SR, Hirose T, Hashida Y et al (2018) Genetic evidence for the role of a rice vacuolar invertase as a molecular sink strength determinant. Rice 11:6
Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51:1126–1136
Okushima Y, Fukaki H, Onoda M et al (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19:118–130
Perez-Riverol Y, Csordas A, Bai J et al (2018) The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47:D442–D450
Pogorelko GV, Mokryakova M, Fursova OV et al (2014) Characterization of three Arabidopsis thaliana immunophilin genes involved in the plant defense response against Pseudomonas syringae. Gene 538:12–22
Romano P, He Z, Luan S (2004) Introducing immunophilins. From organ transplantation to plant biology. Plant Physiol 134:1241–1243
Ruan YL (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65:33–67
Santos Teixeira JA, Tusscher KH (2019) The systems biology of lateral root formation: connecting the dots. Mol Plant 12:784–803
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Smyczynski C, Roudier F, Gissot L et al (2006) The C terminus of the immunophilin pasticcino1 is required for plant development and for interaction with a nac-like transcription factor. J Biol Chem 281:25475–25484
Somssich M, Khan GA, Persson S (2016) Cell wall heterogeneity in root development of Arabidopsis. Front Plant Sci 7:1242
Sun CH, Yu JQ, Hu DG (2017) Nitrate: A crucial signal during lateral roots development. Front Plant Sci 8:485
Tian H, De Smet I, Ding Z (2014) Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci 19:426–431
Vasudevan D, Gopalan G, Kumar A et al (2015) Plant immunophilins: a review of their structure-function relationship. Biochim Biophys Acta 1850:2145–2158
Wang L, Li XR, Lian H et al (2010) Evidence that high activity of vacuolar invertase is required for cotton fiber and Arabidopsis root elongation through osmotic dependent and independent pathways, respectively. Plant Physiol 154:744–756
Wang B, Bailly A, Zwiewka M et al (2013) Arabidopsis TWISTED DWARF1 functionally interacts with auxin exporter ABCB1 on the root plasma membrane. Plant Cell 25:202–214
Wei Z, Li J (2016) Brassinosteroids regulate root growth, development, and symbiosis. Mol Plant 9:86–100
Winter D, Vinegar B, Nahal H et al (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2:e718
Xie C, Mao X, Huang J et al (2011) KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39:316–322
Xiong Y, Sheen J (2012) Rapamycin and glucose-target of rapamycin (TOR) protein signaling in plants. J Biol Chem 287:2836–2842
Zheng L, Liu G, Meng X et al (2012) A versatile agrobacterium-mediated transient gene expression system for herbaceous plants and trees. Biochem Genet 50:761–769
Acknowledgements
This work was supported by grants from the China Transgenic Program (2019ZX08010-004). The funders were not involved in the design of the study, the data collection and analysis, or the writing of the manuscript. We thank Dr. Sheng Luan (University of California Berkeley) for kindly providing the overexpression vector. We are also grateful to the Instrument Analysis Centre of Shanghai Jiao Tong University for LC-MS/MS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The datasets are in a publicly accessible repository
The datasets analysed for this study can be found under dataset identifiers PXD017200 and 10.6019/PXD017200 in the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org/cgi/GetDataset. Perez-Riverol et al. 2018).
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Sun, W., Kong, X. et al. The peptidyl-prolyl isomerases FKBP15-1 and FKBP15-2 negatively affect lateral root development by repressing the vacuolar invertase VIN2 in Arabidopsis. Planta 252, 52 (2020). https://doi.org/10.1007/s00425-020-03459-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03459-2