Abstract
Main conclusion
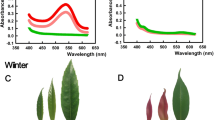
Up to 40% of incident light was screened in red Berberis leaves in vivo by anthocyanins, resulting also in up to 40% reduction of light-limited photosynthesis.
The biological function of anthocyanins in leaves has been strongly discussed, but the hypothesis of a screening function is favored by most authors. For an evaluation of the function as photoprotective pigments, a quantification of their screening of the mesophyll is important. Here, chlorophyll fluorescence excitation of leaves of a red and a green variety of Berberis thunbergii was used to estimate the extent of screening by anthocyanins at 545 nm and over the whole photosynthetically active wavelength range. Growth at high light (430 µmol m−2 s−1) resulted in 90% screening at 545 nm corresponding to 40–50% screening over the whole wavelength range, depending on the light source. The concomitant reduction of photosynthetic quantum yield was of the same size as the calculated reduction of light reaching the chloroplasts. The induction of anthocyanins in the red variety also enhanced the epoxidation state of the violaxanthin cycle under growth conditions, indicating that red leaves were suffering less from excessive irradiance. Pool sizes of violaxanthin cycle carotenoids indicated a shade acclimation of the light harvesting complexes in red leaves. The observed reduction of internal light in anthocyanic leaves has by necessity a photoprotective effect.










Similar content being viewed by others
Abbreviations
- EPS:
-
Epoxidation state of the violaxanthin cycle
- F 545 (F 650):
-
Chlorophyll fluorescence excited by 545 nm (650 nm)
- PAR:
-
Photosynthetically active radiation
- PPFD:
-
Photosynthetic photon flux density
References
Agati G, Pinelli P, Cortés Ebner S, Romani A, Cartelat A, Cerovic ZG (2005) Nondestructive evaluation of anthocyanins in olive (Olea europaea) fruits by in situ chlorophyll fluorescence spectroscopy. J Agric Food Chem 53:1354–1363
Agati G, Meyer S, Matteini P, Cerovic ZG (2007) Assessment of anthocyanins in grape (Vitis vinifera L.) berries using a noninvasive chlorophyll fluorescence method. J Agric Food Chem 55:1053–1061
Archetti M (2009) Classification of hypotheses on the evolution of autumn colours. Oikos 118:328–333
Aronsson H, Jarvis P (2002) A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett 529:215–220
Asen S, Norris KH, Stewart RN (1969) Absorption spectra and color of aluminium-cyanidin 3-glucoside complexes as influenced by pH. Phytochemistry 8:653–659
Asen S, Stewart RN, Norris KH (1972) Co-pigmentation of anthocyanins in plant tissues and its effect on color. Phytochemistry 11:1139–1144
Bilger W, Lesch M (1995) The epoxidation state of the violaxanthin cycle is linearly correlated with photosystem II quantum yield under natural conditions. In: Mathis P (ed) Photosynthesis: from light to biosphere. Kluwer Academic, Dordrecht, pp 107–110
Bilger W, Veit M, Schreiber L, Schreiber U (1997) Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol Plant 101:754–763
Bilger W, Johnsen T, Schreiber U (2001) UV-excited chlorophyll fluorescence as a tool for the assessment of UV-protection by the epidermis of plants. J Exp Bot 52:2007–2014
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170:489–504
Burger J, Edwards GE (1996) Photosynthetic efficiency, and photodamage by UV and visible radiation in red versus green leaf coleus varieties. Plant Cell Physiol 37:395–399
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
Christie PJ, Alfenito MR, Walbot V (1994) Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194:541–549
Close DC, Beadle CL (2003) The ecophysiology of foliar anthocyanins. Bot Rev 69:149–161
Demmig-Adams B, Adams WW (1992) Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ 15:411–419
Esteban R, Fernández-Marín B, Becerril JM, García-Plazaola JI (2008) Photoprotective implications of leaf variegation in E. dens-canis L. and P. officinalis L. J Plant Physiol 165:1255–1263
Feild TS, Lee DW, Holbrook M (2001) Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of Red-Osier Dogwood. Plant Physiol 127:566–574
Fondom NY, Castro-Nava S, Huerta AJ (2014) Field assessment of sub-epidermal anthocyanin, PSII photochemistry, and the xanthophyll-cycle as photoprotective mechanisms in two morphs of Agave striata. Flora 209:131–141
Gamon JA, Peñuelas J, Field CB (1992) A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens Environ 41:35–44
Gates DM (1980) Biophysical ecology. Springer, Berlin
Gitelson AA, Merzlyak MN, Chivkunova OB (2001) Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem Photobiol 74:38–45
Giusti MM, Rodríguez-Saona LE, Wrolstad RE (1999) Molar absorptivity and color characteristics of acylated and non-acylated pelargonidin-based anthocyanins. J Agric Food Chem 47:4631–4637
Gong Z, Yamazaki M, Sugiyama M, Tanaka Y, Saito K (1997) Cloning and molecular analysis of structural genes involved in anthocyanin biosynthesis and expressed in a forma-specific manner in Perilla frutescens. Plant Mol Biol 35:915–927
Gould KS (2004) Nature’s swiss army knife: the diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol 5:314–320
Gould KS, Quinn BD (1999) Do anthocyanins protect leaves of New Zealand native species from UV-B? N Z J Bot 37:175–178
Gould KS, McKelvie J, Markham KR (2002a) Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ 25:1261–1269
Gould KS, Vogelmann TC, Han T, Clearwater MJ (2002b) Profiles of photosynthesis within red and green leaves of Quintinia serrata. Physiol Plant 116:127–133
Hagen SF, Solhaug KA, Bengtsson GB, Borge GIA, Bilger W (2006) Chlorophyll fluorescence as a tool for non-destructive estimation of anthocyanins and total flavonoids in apples. Postharvest Biol Technol 41:156–163
Hasegawa H, Fukasawa-Akada T, Okuno T, Niizeki M, Suzuki M (2001) Anthocyanin accumulation and related gene expression in Japanese parsley (Oenanthe stolonifera, DC.) induced by low temperature. J Plant Physiol 158:71–78
Hatier J-HB, Clearwater MJ, Gould KS (2013) The functional significance of black-pigmented leaves: photosynthesis, photoprotection and productivity in Ophiopogon planiscapus ‘Nigrescens’. PLoS ONE 8:e67850
Hughes NM (2011) Winter leaf reddening in ‘evergreen’ species. New Phytol 190:573–581
Hughes NM, Ley-Yadun S (2015) Red/purple leaf margin coloration: potential ecological and physiological functions. Environ Exp Bot 119:27–39
Hughes NM, Neufeld HS, Burkey KO (2005) Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol 168:575–587
Hughes NM, Morley CB, Smith WK (2007) Coordination of anthocyanin decline and photosynthetic maturation in juvenile leaves of three deciduous tree species. New Phytol 175:675–685
Hughes NM, Burkey KO, Cavender-Bares J, Smith WK (2011) Xanthophyll cycle pigment and antioxidant profiles of winter-red (anthocyanic) and winter-green (acyanic) angiosperm evergreen species. J Exp Bot 63:1895–1905
Inada K (1976) Action spectra for photosynthesis in higher plants. Plant Cell Physiol 17:355–365
Jay-Allemand C, Tattini M, Gould KS (2015) New evidence fort the functional roles of secondary metabolites in plant–environment interactions: special issue of environmental and experimental botany (EEB). Environ Exp Bot 119:1–3
Kimura M, Yamamoto YY, Seki M, Sakurai T, Sato M, Abe T, Yoshida S, Manabe K, Shinozaki K, Matsui M (2003) Indentification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem Photobiol 77:226–233
Kolb CA, Pfündel EE (2001) Origins of non-linear and dissimilar relationships between epidermal UV absorbance and UV absorbance of extracted phenolics in leaves of grapevine and barley. Plant Cell Environ 28:580–590
Kondo T, Yoshida K, Nakagawa A, Kawai T, Tamura H, Goto T (1992) Structural basis of blue-colour development in flower petals from Commelina communis. Nature 358:515–518
Kovinich N, Kayanja G, Chanoca A, Riedl K, Otegui MS, Grotewold E (2014) Not all anthocyanins are born equal: distinct patterns induced by stress in Arabidopsis. Planta 240:931–940
Kyparissis A, Grammatikopoulos G, Manetas Y (2007) Leaf morphological and physiological adjustments to the spectrally selective shade imposed by anthocyanins in Prunus cerasifera. Tree Physiol 27:849–857
Kytridis VP, Manetas Y (2006) Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: evidence linking the putative antioxidant role to the proximity of oxy-radical source. J Exp Bot 57:2203–2210
Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant–environment interactions. Environ Exp Bot 119:4–17
Lea US, Slimestad R, Smedvig P, Lillo C (2007) Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta 225:1245–1253
Lee DW, Collins TM (2001) Phylogenetic and ontogenetic influences on the distribution of anthocyanins and betacyanins in leaves of tropical plants. Int J Plant Sci 162:1141–1153
Lehrer JM, Brand MH (2010) Purple-leaved Japanese barberry (var. atropurpurea) genotypes become visually indistinguishable from green-leaved genotypes (Berberis thunbergii DC.) at low light levels. J Environ Hortic 28:187–189
Logan BA, Stafstrom WC, Walsh MJL, Reblin JS, Gould KS (2015) Examining the photoprotection hypothesis for adaxial foliar anthocyanin accumulation by revisiting comparisons of green- and red-leafed varieties of coleus (Solenostemon scutellarioides). Photosynth Res 124:267–274
Manetas Y (2006) Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora 20:163–177
Manetas Y, Buschmann C (2011) The interplay of anthocyanin biosynthesis and chlorophyll catabolism in senescing leaves and the question of photosystem II photoprotection. Photosynthetica 49:515–522
Manetas Y, Drinia A, Petropoulou Y (2002) High contents of anthocyanins in young leaves are correlated with low pools of xanthophyll cycle components and low risk of photoinhibition. Photosynthetica 40:349–355
Manetas Y, Petropoulou Y, Psaras GK (2003) Exposed red (anthocyanic) leaves of Quercus coccifera display shade characteristics. Funct Plant Biol 30:265–270
McCree KJ (1972) The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric Meteorol 9:191–216
Nichelmann L, Schulze M, Herppich WB, Bilger W (2016) A simple indicator for non-destructive estimation of the violaxanthin cycle pigment content in leaves. Photosynth Res 128:183–193
Nielsen SI, Simonsen AM (2011) Photosynthesis and photoinhibition in two differently coloured varieties of Oxalis triangularis—the effect of anthocyanin content. Photosynthetica 49:346–352
Olsen KM, Slimestad R, Lea US, Brede C, Løvdal T, Ruoff P, Verheul M, Lillo C (2009) Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant Cell Environ 32:286–299
Pfündel EE, Ben Ghozlen N, Meyer S, Cerovic ZG (2007) Investigating UV screening in leaves by two different types of portable UV fluorimeters reveals in vivo screening by anthocyanins and carotenoids. Photosynth Res 93:205–221
Pietrini F, Iannelli MA, Massacci A (2002) Anthocyanin accumulation in the illuminated surface of maize leaves enhances protection from photo-inhibitory risks at low temperature, without further limitation to photosynthesis. Plant Cell Environ 25:1251–1259
Porra RL, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Rapisarda P, Fanella F, Maccarone E (2000) Reliability of analytical methods for determining anthocyanins in blood orange juices. J Agric Food Chem 48:2249–2252
Rowan DD, Cao M, Lin-Wang K, Cooney JM, Jensen DJ, Austin PT, Hunt MB, Norling C, Hellens RP, Schaffer RJ, Allan AC (2009) Environmental regulation of leaf colour in red 35S:PAP1 Arabidopsis thaliana. New Phytol 182:102–115
Sharp RE, Matthews MA, Boyer JS (1984) Kok effect and the quantum yield of photosynthesis. Plant Physiol 75:95–101
Smillie RM, Hetherington SE (1999) Photoabatement by anthocyanin shields photosynthetic systems from light stress. Photosynthetica 36:451–463
Steyn WJ, Wand SJE, Holcroft DM, Jacobs G (2002) Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 155:349–361
Tattini M, Landi M, Brunetti C, Giordano C, Remorini D, Gould KS, Guidi L (2014) Epidermal coumaroyl anthocyanins protect sweet basil against excess light stress: multiple consequences of light attenuation. Physiol Plant 152:585–598
Tattini M, Sebastiani F, Brunetti C, Fini A, Torre S, Gori A, Centritto M, Ferrini F, Landi M, Guidi L (2017) Dissecting molecular and physiological response mechanisms to high solar radiation in cyanic and acyanic leaves: a case study on red and green basil. J Exp Bot 68:2425–2437
Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:331–343
Torre S, Tattini M, Brunetti C, Guidi L, Gori A, Marzano C, Landi M, Sebastiani F (2016) De novo assembly and comparative transcriptome analyses of red and green morphs of sweet basil grown in full sunlight. PLoS ONE 11:e0160370
Tyystjärvi E, Aro EM (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93:2213–2218
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Zhang KM, Yu HJ, Shi K, Zhou YH, Yu JQ, Xia XJ (2010) Photoprotective roles of anthocyanins in Begonia semperflorens. Plant Sci 179:202–208
Acknowledgements
Anneke Kramm and Jens Hermann are thanked for help with pigment determination.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nichelmann, L., Bilger, W. Quantification of light screening by anthocyanins in leaves of Berberis thunbergii . Planta 246, 1069–1082 (2017). https://doi.org/10.1007/s00425-017-2752-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2752-2