Abstract
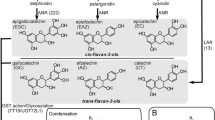
Proanthocyanidins (PAs) are oligomers or polymers of plant flavan-3-ols and are important to plant adaptation in extreme environmental conditions. The characterization of anthocyanidin reductase (ANR) and leucoanthocyanidin reductase (LAR) has demonstrated the different biogenesis of four stereo-configurations of flavan-3-ols. It is important to understand whether ANR and the ANR pathway widely occur in the plant kingdom. Here, we report an integrated approach to demonstrate the ANR pathway in plants. This includes different methods to extract native ANR from different tissues of eight angiosperm plants (Lotus corniculatus, Desmodium uncinatum, Medicago sativa, Hordeum vulgare, Vitis vinifera, Vitis bellula, Parthenocissus heterophylla, and Cerasus serrulata) and one fern plant (Dryopteris pycnopteroides), a general enzymatic analysis approach to demonstrate the ANR activity, high-performance liquid chromatography-based fingerprinting to demonstrate (−)-epicatechin and other flavan-3-ol molecules, and phytochemical analysis of PAs. Results demonstrate that in addition to leaves of M. sativa, tissues of other eight plants contain an active ANR pathway. Particularly, the leaves, flowers and pods of D. uncinatum, which is a model plant to study LAR and the LAR pathways, are demonstrated to express an active ANR pathway. This finding suggests that the ANR pathway involves PA biosynthesis in D. uncinatum. In addition, a sequence BLAST analysis reveals that ANR homologs have been sequenced in plants from both gymnosperms and angiosperms. These data show that the ANR pathway to PA biosynthesis occurs in both seed and seedless vascular plants.








Similar content being viewed by others
Abbreviations
- ANR:
-
Anthocyanidin reductase
- HPLC:
-
High-performance liquid chromatography
- TLC:
-
Thin layer chromatography
- PA:
-
Proanthocyanidins
References
Alonso-Amelot ME, Oliveros A, Calcagno-Pisarelli MP (2004) Phenolics and condensed tannins in relation to altitude in neotropical Pteridium spp. A field study in the Venezuelan Andes. Biochem Syst Ecol 32:969–981
Alonso-Amelot ME, Oliveros-Bastidas A, Calcagno-Pisarelli MP (2007) Phenolics and condensed tannins of high altitude Pteridium arachnoideum in relation to sunlight exposure, elevation, and rain regime. Biochem Syst Ecol 35:1–10
Ariga T (2004) The antioxidative function, preventive action on disease and utilization of proanthocyanidins. BioFactors 21:197–201
Bailey JK, Schweitzer JA, Rehill BJ, Lindroth RL, Martinsen GD, Whitham TG (2004) Beavers as molecular geneticists: a genetic basis to the foraging of an ecosystem engineer. Ecology 85:603–608
Bernays EA (1981) Plant tannins and insect herbivores-An appraisal. Ecol Entomol 6:353–360
Bogs J, Downey MO, Harvey JS, Ashton AR, Tanner GJ, Robinson SP (2005) Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol 139:652–663
Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson SP (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol 143:1347–1361
Choi YT, Jung CH, Lee SR, Bae JH, Baek WK, Suh MH, Park J, Park CW, Suh SI (2001) The green tea polyphenol (−)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci 70:603–614
Constantin Z (1841) Ueber Catechin. Annalen der Chemie und Pharmacie 37:320–336
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard J-F, Guindon S, Lefort V, Lescot M, Claverie J-M, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:W465–W469
Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins: a final frontier in flavonoid research? New Phytol 165:9–28
Ferreira D, Slade D (2002) Oligomeric proanthocyanidins: naturally occuring O-heterocycles. Nat Prod Rep 19:517–541
Foo LY, Newman R, Waghorn G, McNabb WC, Ulyatt MJ (1996) Proanthocyanidins from Lotus corniculatus. Phytochemistry 41:617–624
Forkner RE, Marquis RJ, Lill JT (2004) Feeny revisited: condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus. Ecol Entomol 29:174–187
Friedrich R (1869) Ueber Catechin und Catechugerbstoff. J für Praktische Chemie 106:307–310
Gagné S, Lacampagne S, Claisse O, Gény L (2009) Leucoanthocyanidin reductase and anthocyanidin reductase gene expression and activity in flowers, young berries and skins of Vitis vinifera L. cv Cabernet-Sauvignon during development. Plant Physiol Biochem 47:282–290
Gardner RO (1977) Systematic distribution and ecological function of the secondary metabolites of the Rosidae-Asteridae. Bioch Syst Ecol 5:29–35
Gargouri M, Gallois B, Chaudiere J (2009a) Binding-equilibrium and kinetic studies of anthocyanidin reductase from Vitis vinifera. Arch Biochem Biophys 491:61–68
Gargouri M, Manigand C, Mauge C, Granier T, Langlois d’Estaintot B, Cala O, Pianet I, Bathany K, Chaudiere J, Gallois B (2009b) Structure and epimerase activity of anthocyanidin reductase from Vitis vinifera. Acta Crystallogr Sect D-Biol Crystallogr 65:989–1000
Gargouri M, Chaudiere J, Manigand C, Mauge C, Bathany K, Schmitter JM, Gallois B (2010) The epimerase activity of anthocyanidin reductase from Vitis vinifera and its regiospecific hydride transfers. Biol Chem 391:219–227
Haslam E (1977) Symmetry and promiscuity in procyanidin biochemistry. Phytochemistry 16:1625–1640
Heil M, Baumann B, Andary C, Linsenmair KE, McKey D (2002) Extraction and quantification of “condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 89:519–524
Hoshizaki BJ, Wilson KA (1999) The cultivated species of the fern genus Dryopteris in the United States. American Fern Journal 89:1–98
Jd Chemie (1865) Ueber das Catechu und das Catechin. Annalen der Chemie und Pharmacie 134:118–122
Kim H (2005) New nutrition, proteomics, and how both can enhance studies in cancer prevention and therapy. J Nutr 135:2715–2718
Kristiansen KN (1984) Biosynthesis of proanthocyanidins in barley: genetic control of the conversion of dihydroquercetin to catechin and procyanidins. Carlsberg Res Commun 49:503–524
Kristiansen KN (1986) Conversion of (+)-dihydroquercetin to (+)-2,3-trans-3,4-cis-leucocyanidin and (+)-catechin with an enzyme extract from maturing grains of barley. C. Carlsberg Res Commun 51:51–60
Lavola A (1998) Accumulation of flavonoids and related compounds in birch induced by UV-B irradiance. Tree Physiol 18:53–58
Lees GL (1992) Condensed tannins in some forage legumes: their role in the prevention of ruminant pasture bloat. Basic Life Sci 59:915–934
Leigh MJ (2003) Health benefits of grape seed proanthocyanidin extract (GSPE). Nutr Noteworthy 6:1–5
Li C-X, Lu S-G (2006) Phylogenetics of Chinese Dryopteris (Dryopteridaceae) based on the chloroplast rps4-trnS sequence data. J Plant Res 119:589–598
Marles MAS, Ray H, Gruber MY (2003) New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry 64:357–383
Miranda M, Ralph SG, Mellway R, White R, Heath MC, Bohlmann J, Constabel CP (2007) The transcriptional response of hybrid poplar (Populus trichocarpa x P-deltoides) to infection by Melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins. Mol Plant-Microbe Interact 20:816–831
Moore MJ, Dhingra A, Soltis PS, Shaw R, Farmerie WG, Folta KM, Soltis DE (2006) Rapid and accurate pyrosequencing of angiosperm plastid genomes. BMC Plant Biol 6:17
Pang Y, Peel GJ, Wright E, Wang Z, Dixon RA (2007) Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol 145:601–615
Pang Y, Peel GJ, Sharma SB, Tang Y, Dixon RA (2008) A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. PNAS 105:14210–14215
Pfeiffer J, Kuhnel C, Brandt J, Duy D, Punyasiri PAN, Forkmann G, Fischer TC (2006) Biosynthesis of flavan 3-ols by leucoanthocyanidin 4-reductases and anthocyanidin reductases in leaves of grape (Vitis vinifera L.), apple (Malus x domestica Borkh.) and other crops. Plant Physiol Biochem 44:323–334
Porter LJ (1988) Flavans and proanthocyanidins. In: Harborne JB (ed) The flavonoids. Chapman and Hall, London, pp 21–62
Porter LJ (1993) Flavans and proanthocyanidins. In: Harborne JB (ed) The flavonoids: advances in research since 1986. Chapman and Hall, London, pp 23–55
Porter LJ (1998) Flavans and Proanthocyanidins. In: Harborne JB (ed) The Flavonoids. Chapman and Hall Ltd., London, pp 21–62
Punyasiri PAN, Abeysinghe ISB, Kumar V, Treutter D, Duy D, Gosch C, Martens S, Forkmann G, Fischer TC (2004) Flavonoid biosynthesis in the tea plant Camellia sinensis: properties of enzymes of the prominent epicatechin and catechin pathways. Arch Biochem Biophys 431:22–30
Rasmussen SE, Frederiksen H, Struntze K, Poulsen KL (2005) Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol Nutrit Food Res 49:159–174
Ray H, Yu M, Auser P, Blahut-Beatty L, McKersie B, Bowley S, Westcott N, Coulman B, Lloyd A, Gruber MY (2003) Expression of anthocyanins and proanthocyanidins after transformation of alfalfa with maize Lc. Plant Physiol 132:1448–1463
Shen GA, Pang YZ, Wu WS, Liu XF, Zhao LX, Sun XF, Tang KX (2006) Isolation and characterization of a putative anthocyanidin reductase gene from Ginkgo biloba. J Plant Physiol 163:224–227
Skadhauge B, Gruber MY, Thomsen KK, von Wettstein D (1997) Leucocyanidin reductase activity and accumulation of proanthocyanidins in developing legume tissues. Am J Botany 84:494–502
Stafford HA (1983) Enzymic regulation of procyanidin biosynthesis: lack of a flav-3-en-ol intermediate. Phytochemistry 22:2643–2646
Stafford HA (1990) Pathway to proanthocyanidins (condensed tannins), flavan-3-ols, and unsubstituted flavans. In: Stafford HA (ed) Flavonoid metabolism. CRC Press, Boca Raton, pp 63–99
Stafford HA, Lester HH (1984) Flavan-3-ol biosynthesis. The conversion of (+)-dihydroquercetin and flavan-3,4-cis-diol (leucocyanidin) to (+)-catechin by reductases extracted from cell suspension cultures of Douglas fir. Plant Physiol 76:184–186
Stafford HA, Lester HH (1985) Flavan-3-ol biosynthesis. The conversion of (+)-dihydromyrecetin to its flavan-3,4-diol (leucodelphinidin) and to (+)-gallocatechin by reductases extracted from tissue cultures of Ginko biloba and Pseudotsuga menziesii. Plant Physiol 78:791–794
Stafford HA, Shimamoto M, Lester HH (1982) Incorporation of [14C]phenylalanine into flavan-3-ols and procyanidins in cell suspension cultures of Douglas Fir. Plant Physiol 69:1055–1059
Svobodová A, Psotová J, Walterová D (2003) Natural phenolics in the prevention of UV-induced skin damage. Biomed 147:137–145
Takahashi A, Ichihara Y, Isagi Y, Shimada T (2010) Effects of acorn tannin content on infection by the fungus Ciboria batschiana. Forest Pathol 40:96–99
Takos AM, Ubi BE, Robinson SP, Walker AR (2006) Condensed tannin biosynthesis genes are regulated separately from other flavonoid biosynthesis genes in apple fruit skin. Plant Sci 170:487–499
Tamagawa K, Fukushima S, Kobori M, Shinmoto H, Tsushida T (1998) Proanthocyanidins from barley bran potentiate retinoic acid-induced granulocytic and sodium butyrate-induced monocytic differentiation of HL60 cells. Biosci Biotechnol Biochem 62:1483–1487
Tanner GJ, Francki KT, Abrahams S, Watson JM, Larkin PJ, Ashton AR (2003) Proanthocyanidin biosynthesis in plants. Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J Biol Chem 278:31647–31656
Tegelberg R, Julkunen-Tiitto R, Aphalo PJ (2001) The effects of long-term elevated UV-B on the growth and phenolics of field-grown silver birch (Betula pendula). Global Change Biol 7:839–848
Waghorn GC, McNabb WC (2003) Consequences of plant phenolic compounds for productivity and health of ruminants. Proc Nutr Soc 62:383–392
Wicker T, Schlagenhauf E, Graner A, Close TJ, Keller B, Stein N (2006) 454 sequencing put to the test using the complex genome of barley. BMC Genomics 7:275
Xie D-Y, Dixon RA (2005) Proanthocyanidin biosynthesis—still more questions than answers? Phytochemistry 66:2127–2144
Xie D-Y, Sharma SB, Paiva NL, Ferreira D, Dixon RA (2003) Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 299:396–399
Xie D-Y, Sharma SB, Dixon RA (2004) Anthocyanidin reductases from Medicago truncatula and Arabidopsis thaliana. Arch Biochem Biophys 422:91–102
Xie D-Y, Sharma SB, Wright E, Wang Z-Y, Dixon RA (2006) Metabolic engineering of proanthocyanidins through co-expression of anthocyanidin reductase and the PAP1 MYB transcription factor. Plant J 45:895–907
Yamaguchi H, Yoshino K (2001) Influence of tannin–copper complexes as preservatives for wood on mechanism of decomposition by brown-rot fungus Fomitopsis palustris. Holzforschung 55:464–470
Zhao J, Dixon RA (2009) MATE transporters facilitate vacuolar uptake of epicatechin 3′-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 21:2323–2340
Zhao J, Pang Y, Dixon RA (2010) The mysteries of proanthocyanidin transport and polymerization. Plant Physiol 153:437–443
Acknowledgments
This work is dedicated to Dr. Richard A. Dixon for his 60th birthday celebration. This work was firstly initiated at Noble Foundation and then continued by supports from Bai-Ren-Ji-Hua’s startup of Jishou University, Hunan, P.R. China, and North Carolina State University startup.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
S-Fig. 1 DMACA staining of D. uncinatum flowers. a Two examples of flowers treated with buffer without DMACA; b two examples of flowers showing deep blue color after treated 2 min with 0.1 % DMACA (g/ml) in buffer. The blue coloration results from reaction of DMACA and proanthocyanidins.
S-Fig. 2 HPLC analysis of catechin and epicatechin from leaves of Dryopteris pycnopteroides; a an extract of D. pycnopteroides leaves; b authentic standards; abbreviation, CA: catechin, EP: epicatechin).
Rights and permissions
About this article
Cite this article
Peng, QZ., Zhu, Y., Liu, Z. et al. An integrated approach to demonstrating the ANR pathway of proanthocyanidin biosynthesis in plants. Planta 236, 901–918 (2012). https://doi.org/10.1007/s00425-012-1670-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-012-1670-6