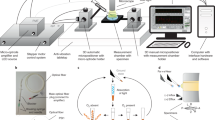
Abstract
The ability to non-invasively measure metabolic oxygen flux is a very important tool for physiologists interested in a variety of questions ranging from basic metabolism, growth/development, and stress adaptation. Technologies for measuring oxygen concentration near the surface of cells/tissues include electrochemical and optical techniques. A wealth of knowledge was gained using these tools for quantifying real-time physiology. Fiber-optic microprobes (optrodes) have recently been developed for measuring oxygen in a variety of biomedical and environmental applications. We have adopted the use of these optical microsensors for plant physiology applications, and used the microsensors in an advanced sensing modality known as self-referencing. Self-referencing is a non-invasive microsensor technique used for measuring real-time flux of analytes. This paper demonstrates the use of optical microsensors for non-invasively measuring rhizosphere oxygen flux associated with respiration in plant roots, as well as boundary layer oxygen flux in phytoplankton mats. Highly sensitive/selective optrodes had little to no hysteresis/calibration drift during experimentation, and an extremely high signal-to-noise ratio. We have used this new tool to compare various aspects of rhizosphere oxygen flux for roots of Glycine max, Zea mays, and Phaseolus vulgaris, and also mapped developmentally relevant profiles and distinct temporal patterns. We also characterized real-time respiratory patterns during inhibition of cytochrome and alternative oxidase pathways via pharmacology. Boundary layer oxygen flux was also measured for a phytoplankton mat during dark:light cycling and exposure to pharamacological inhibitors. This highly sensitive technology enables non-invasive study of oxygen transport in plant systems under physiologically relevant conditions.






Similar content being viewed by others
References
Alderman J, Hynes J, Floyd SM, Kruger J, O’Connor R, Papkovsky DB (2004) A low-volume platform for cell-respirometric screening based on quenched-luminescence oxygen sensing. Biosens Bioelectron 19(11):1529–1535
Armstrong W, Webb T, Darwent M, Beckett PM (2009) Measuring and interpreting respiratory critical oxygen pressures in roots. Ann Botany 103:281–293
Arnholdt-Schmitt B, Costa JH, de Melo DF (2006) Aox—a functional marker for efficient cell reprogramming under stress? Trends Plant Sci 11:281–287
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Ann Rev Plant Biol 59:313–339
Borgens RB (1984) Endogenous ionic currents traverse intact and damaged bone. Science 225:478–482
Borisov SM, Wolfbeis OS (2008) Optical biosensors. Chem Rev 108:423–461
Buerk DG (2004) Oxygen sensing. Meth Enzymol 381:665–690
Chatni MR, Porterfield DM (2009) Self-referencing optrode technology for non-invasive real-time measurement of biophysical flux and physiological sensing. Analyst 134:2224–2232
Chatni MR, Maier DE, Porterfield DM (2009) Optimization of oxygen sensitive optical dye membrane polymers for fluorescent lifetime based physiological biosensing. Sens Actuat B Chem 141:471–477
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala SN (2005) Screening plants for salt tolerance by measuring K+ flux; a case study for barley. Plant Cell Environ 28:1230–1246
Cloutier M, Chen J, Tatge F, McMurray-Beaulieu V, Perriera M, Jolicoeur M (2009) Kinetic metabolic modelling for the control of plant cells cytoplasmic phosphate. J Theor Biol 259(1):118–131
Darwent MJ, Armstrong W, Armstrong J, Beckett PM (2003) Exploring the radial and longitudinal aeration of primary maize roots by means of clark-type oxygen microelectrodes. Russ J Plant Physiol 50(6):722–732
De Dobbeleer C, Cloutier M, Fouilland M, Legros R, Jolicoeur MA (2006) High-rate perfusion bioreactor for plant cells. Biotechnol Bioeng 95(6):1126–1137
Dodds WK, Biggs BJF, Lowe RL (1999) Photosynthesis-irradiance patterns in benthic microalgae: variations as a function of assemblage thickness and community structure. J Phycol 35(1):42–53
Feijo JA, Sainhas J, Holdaway-Clarke T, Cordeiro MS, Kunkel JG, Hepler PK (2001) Cellular oscillations and the regulation of growth: the pollen tube paradigm. Bioassays 23:86–94
Ferris MJ, Hirsch CF (1991) Method for isolation and purification of cyanobacteria. Appl Environ Microbiol 57(5):1448–1452
Gilliham M, Sullivan W, Tester M, Tyerman SD (2006) Simultaneous flux and current measurement from single plant protoplasts reveals a strong link between K+ fluxes and current, but no link between Ca2 + fluxes and current. Plant J 46:134–144
Gradmann D, Slayman CL (1975) Oscillations of an electrogenic pump in the plasma membrane of Neurospora. J Membr Biol 23:181–212
Gupta KJ, Zabalza A, van Dongen JT (2009) Regulation of respiration when the oxygen availability changes. Physiol Plant 137:383–391
Holdaway-Clarke TL, Hepler PK (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytol 159:539–563
Jaffe LF, Nuccitelli R (1974) An ultrasensitive vibrating probe for measuring steady extracellular currents. J Cell Biol 63:614–628
Kochian LV, Shaff JE, Kühtreiber WM, Jaffe LF, Lucas WJ (1992) Use of an extracellular, ion-selective, vibrating microelectrode system for the quantification of K+, H+, and Ca2+ fluxes in maize roots and maize suspension cells. Planta 188:601–610
Kühl M, Jørgensen BB (1992) Spectral light measurements in microbenthic phototrophic communities with a fiber-optic microprobe coupled to a sensitive diode array detector. Limnol Oceanogr 37:1813–1823
Kühtreiber WM, Jaffe LF (1990) Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J Cell Biol 110:1565–1573
Kupper H, Setlik I, Hlasek M (2004) A versatile chamber for simultaneous measurements of oxygen exchange and fluorescence in filamentous and thallous algae as well as higher plants. Photosynthetica 42(4):579–583
Laan P, Tosserams M, Huys P, Bienfait HF (1991) Oxygen uptake by roots of rumex species at different temperatures—the relative importance of diffusive resistance and enzyme kinetics. Plant Cell Environ 14(2):235–240
Lamboursain L, St-Onge F, Jolicoeur M (2002) A lab-built respirometer for plant and animal cell culture. Biotechnol Prog 18(6):1377–1386
Land SC, Portefield DM, Sanger RH, Smith PJS (1999) The self-referencing oxygen-selective microelectrode: detection of transmembrane oxygen flux from single cells. J Exp Biol 202:211–218
Lee SK, Okura I (1997) Photostable optical oxygen sensing material: platinum tetrakis(pentafluorophenyl)porphyrin immobilized in polystyrene. Anal Comm 34(6):185–188
Mancoso S, Boselli M (2002) Characterisation of the oxygen fluxes in the division, elongation and mature zone of Vitis roots: influence of oxygen availability. Planta 214:767–774
Mancuso S, Papeschi G, Marras AM (2000) A polarographic, oxygen-selective, vibrating-microelectrode system for the spatial and temporal characterization of transmembrane oxygen fluxes in plants. Planta 211:384–389
McLamore ES, Porterfield DM, Banks MK (2009) Non-invasive self-referencing electrochemical sensors for quantifying real time biophysical flux in biofilms. Biotechnol Bioeng 102:791–799
McLamore ES, Mohanty S, Shi S, Claussen J, Jedlicka SS, Rickus JL, Porterfield DM (2010a) A self-referencing glutamate biosensor for measuring real time neuronal glutamate flux. J Neurosci Methods 189:14–22
McLamore ES, Stensberg M, Yale G, Ochoa-Acuna H, Sepulveda M, Sun X, Akkus O, Porterfield DM (2010b) A difference imaging technique for monitoring real time changes in morphology within the cell, tissue, and organism spatial domain. Proc SPIE 7674(14):1–9
Mclntosh L (1994) Molecular biology of the alternative oxidase. Plant Physiol 105:781–786
Miller HL, Dunton KH (2007) Stable isotope (C-13) and O2 micro-optode alternatives for measuring photosythesis in seaweeds. Mar Ecol Prog Ser 329:85–97
O’Riordan TC, Buckley D, Ogurtsov VI, O’Connor R, Papkovsky DB (2000) A cell viability assay based on monitoring respiration by optical oxygen sensing. Anal Biochem 278:221–227
Ober ES, Sharp RE (1996) A microsensor for direct measurement of O2 partial pressure within plant tissues. J Exp Bot 47(296):447–454
Polidoros AN, Mylona PV, Arnholdt-Schmitt B (2009) Aox gene structure, transcript variation and expression in plants. Physiol Plant 137(4):342–353
Porterfield DM (2002) The use of microsensors for studying the physiological activity of plant roots. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Marcel Decker, New York, pp 333–347
Porterfield DM (2007) Measuring metabolism and biophysical flux in the tissue, cellular and sub-cellular domains: recent developments in self-referencing amperometry for physiological sensing. Biosens Bioelectron 22:1186–1196
Porterfield DM, Smith PJS (2000) Single-cell, real-time measurements of extracellular oxygen and proton fluxes from Spirogyra grevilleana. Protoplasma 212:80–88
Porterfield DM, Trimarchi JR, Keefe DL, Smith PJS (1998) Characterization of oxygen and calcium fluxes from early mouse embryos and oocytes. Biol Bull 195:208–209
Porterfield DM, Kuang A, Smith PJS, Crispi ML, Musgrave ME (1999) Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can J Bot 77:1439–1446
Porterfield DM, Corkey RF, Sanger RH, Tornheim K, Smith PJS, Corkey BE (2000) Oxygen consumption oscillates in single clonal pancreatic β-cells (HIT). Diabetes 49:1511–1516
Porterfield DM, Rickus JL, Kopelman R (2007) Non-Invasive approaches to measuring respiratory patterns using a pttfpp based, phase-lifetime, self-referencing oxygen optrode. Proc Soc Photonics (SPIE) 6380:1–8
Porterfield DM, McLamore ES, Banks MK (2009) Microsensor technology for measuring H+ flux in buffered media. Sens Actuat B Chem 136:383–387
Richard P (2003) The rhythm of yeast. FEMS Microbiol Rev 791:1–11
Ryan PR, Ditomaso JM, Kochian LV (1993) Aluminum toxicity in roots—an investigation of spatial sensitivity and the role of the root cap. J Exp Bot 44:437–446
Sanchez BC, Ochoa-Acuña H, Porterfield DM, Sepúlveda MS (2008) Oxygen flux as an indicator of physiological stress in fathead minnow (Pimephales promelas) embryos: a real-time biomonitoring system of water quality. Environ Sci Technol 42:7010–7017
Serrano M, Robatzek S, Torres M, Kombrink E, Somssich IE, Robinson M, Schulze-Lefert P (2007) Chemical interference of pathogen-associated molecular pattern-triggered immune responses in Arabidopsis reveals a potential role for fatty-acid synthase type II complex-derived lipid signals. J Biol Chem 282(9):6803–6811
Shabala SN, Newman IA (1997) Proton and calcium flux oscillations in the elongation region correlate with root nutation. Physiol Plant 100(4):917–926
Shabala SN, Newman IA (1998) Osmotic sensitivity of Ca2+ and H+ transporters in corn roots: effect on fluxes and their oscillations in the elongation region. J Membr Biol 161:45–54
Shabala SN, Shabala L (2002) Kinetics of net H+, Ca2+, K+, Na+, NH4+, and Cl− fluxes associated with post-chilling recovery of plasma membrane transporters in Zea mays leaf and root tissues. Physiol Plant 114:47–56
Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113:111–118
Shabala SN, Newman I, Whittington J, Juswono U (1998) Protoplast ion fuxes: their measurement and variation with time, position and osmoticum. Planta 204:146–152
Shabala L, Ross T, McMeekin T, Shabala S (2006) Non-invasive microelectrode ion flux measurements to study adaptive responses of microorganisms to the environment. FEMS Microbiol Rev 30:472–486
Shi K, Hu WH, Dong DK, Zhou YH, Yu JQ (2007) Low O2 supply is involved in the poor growth in root-restricted plants of tomato (Lycopersicon esculentum Mill.). Environ Exp Bot 61(2):181–189
Siedow JN, Umbach AL (2000) The mitochondrial cyanide-resistant oxidase: structural conservation amid regulatory diversity. Biochim Biophys Acta 1459:432–439
Verslues PE, Ober ES, Sharp RE (1998) Root growth and oxygen relations at low water potentials. Impact of oxygen availability in polyethylene glycol solutions. Plant Physiol 116:1403–1412
Wolfbeis OS (2004) Fiber-optic chemical sensors and biosensors. Anal Chem 76:3269–3284
Zitova A, O’Mahony FC, Cross M, Davenport J, Papkovsky DB (2009) Toxicological profiling of chemical and environmental samples using panels of test organisms and optical oxygen respirometry. Environ Toxicol 24(2):116–127
Zuberi M, Liu-Snyder P, Ul Haque A, Porterfield DM, Borgens RB (2008) Large naturally-produced electric currents and voltage traverse damaged mammalian spinal cord. J Biol Eng 2:17–26
Acknowledgments
The authors would like to thank the National Science Foundation for funding this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McLamore, E.S., Jaroch, D., Chatni, M.R. et al. Self-referencing optrodes for measuring spatially resolved, real-time metabolic oxygen flux in plant systems. Planta 232, 1087–1099 (2010). https://doi.org/10.1007/s00425-010-1234-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1234-6