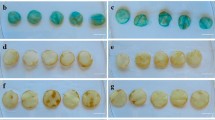
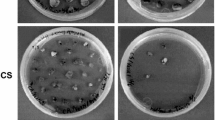
Abstract
The first step of Agrobacterium tumefaciens/plant interaction corresponds to the activation of a transduction pathway of the bacterium by plant exudate. Phenolic compounds rapidly secreted by wounded plant cells induce the expression of bacterial virulence (vir) genes; however, little is known about their biosynthesis in plant. Here we show that inoculation of an Agrobacterium tumefaciens virulent strain on orthodiphenol-O-methyltransferases-suppressed tobacco plants leads to significantly smaller tumors compared to control plants. These transgenic plants are inhibited for caffeic acid O-methyltransferase class I or II (OMT; EC 2.1.1.6) and/or caffeoyl-coenzyme A O-methyltransferase (CCoAOMT; EC 2.1.1.104) that are involved in monolignol biosynthesis. The significant decrease of tumor size could be suppressed by the pre-activation of bacterial virulence, before inoculation, using acetosyringone a known vir inducer. Total soluble phenolic amounts and cell wall composition analyzed by FT-IR analysis did not show significant differences between transgenic and control plants. The potential of phenolic extracts from control and OMT-suppressed plants to induce virulence was evaluated using an Agrobacterium tumefaciens reporter strain carrying a vir::LacZ gene fusion plasmid. Lower vir-inducing activities were recorded for plants that show inhibition to caffeic acid O-methyltransferase activity. HPLC analysis confirmed that the levels of several phenolic compounds were differently affected by wounding and/or by bacterial inoculation. Statistical correlations were established between tumor sizes, vir-inducing activities, O-methyltransferases proteins accumulations and the levels of various soluble phenolic compounds such as acetosyringone. These results demonstrate the role of the O-methyltransferases of the phenylpropanoid pathway in the early production of soluble Agrobacterium tumefaciens vir inducers.






Similar content being viewed by others
Abbreviations
- CCoAOMT:
-
Caffeoyl-coenzyme A O-methyltransferase
- COMTI:
-
Caffeic acid O-methyltransferase of class I
- COMTII:
-
Caffeic acid O-methyltransferase of class II
- FT-IR:
-
Fourier transformed-infrared
- HPLC:
-
High-performance liquid chromatography
- OMT:
-
Orthodiphenol-O-methyltransferases
- Vir :
-
Virulence
References
Anand A, Vaghchhipawala Z, Ryu C-M, Kang L, Wang K, del-Pozo O, Martin GB, Mysore KS (2007) Identification and characterization of plant genes involved in Agrobacterium-mediated plant transformation by virus-induced gene silencing. Mol Plant Microbe Interact 20:41–52
Atanassova R, Favet N, Martz F, Chabbert B, Tollier MT, Monties B, Fritig B, Legrand M (1995) Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J 8:465–477
Baker CJ, Mock NM, Whitaker BD, Roberts DP, Rice CP, Deahl KL, Aver’yanov AA (2005) Involvement of acetosyringone in plant-pathogen recognition. Biochem Biophys Res Com 328:130–136
Berthelot K, Buret D, Guérin B, Delay D, Negrel J, Delmotte FM (1998) Vir-gene-inducing activities of hydroxycinnamic acid amides in Agrobacterium tumefaciens. Phytochemistry 49:1537–1548
Causevic A, Delaunay A, Ounnar S, Righezza M, Delmotte FM, Brignolas F, Hagège D, Maury S (2005) DNA methylating and demethylating treatments modify phenotype and cell wall differentiation state in sugarbeet cell lines. Plant Physiol Biochem 43:681–691
Chabannes M, Barakate A, Lapierre C, Marita JM, Ralph J, Pean M, Danoun S, Halpin C, Grima-Pettenati J, Boudet AM (2001) Strong alteration in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl coA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant J 28:257–270
Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ, Ron E, Faure D (2006) GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 103:7460–7464
Dafny-Yelin M, Levy A, Tzfira T (2008) The ongoing saga of Agrobacterium–host interactions. Trends Plant Sci 13:102–105
De Ascensao AR, Dubery IA (2003) Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f.sp. cubense. Phytochemistry 63:679–686
Ditt RF, Kerr KF, de Figueiredo P, Delrow J, Comai L, Nester EW (2006) The Arabidopsis thaliana transcriptome in response to Agrobacterium tumefaciens. Mol Plant Microbe Interact 19:665–681
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MSS, Wang L (2002) The phenylpropanoid pathway and plant defense-a genomics perspective. Mol Plant Pathol 3:371–390
Do C-T, Pollet B, Thévenin J, Sibout R, Denoue D, Barrière Y, Lapierre C, Jouanin L (2007) Both caffeoyl coenzyme A 3-O-methyltransferase 1 and caffeic acid 3-O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta 226:1117–1129
Doorsselaere JV, Baucher M, Chognot E, Chabbert B, Tollier M-T, Petit-Conil M, Leplé J-C, Pilate G, Cornu D, Monties B, Van Montagu M, Inzé D, Boerjan W, Jouanin L (1995) A novel lignin in poplar trees with a reduced caffeic acid/5-hydroxyferulic acid O-methyltransferase activity. Plant J 8:855–864
Ferrer J-L, Austin MB, Stewart CJ, Noel JP (2008) Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem 46:356–370
Gelvin SB (2006) Agrobacterium virulence gene induction. Methods Mol Biol 343:77–84
Hano C, Addi M, Bensaddek L, Crônier D, Baltora-Rosset S, Doussot J, Maury S, Mesnard F, Chabbert B, Hawkins S, Lainé E, Lamblin F (2006) Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223:975–989
Hawkins S, Boudet AM (2003) Defense lignin and hydroxycinnamyl alcohol dehydrogenase activities in wounded Eucalyptus gunnii hook. Forest Pathol 33:91–104
Hoffmann L, Maury S, Bergdoll M, Thion L, Erard M, Legrand M (2001) Identification of the enzymatic active site of tobacco caffeoyl-coenzyme A O-methyltransferase by site-directed mutagenesis. J Biol Chem 276:36831–36838
Hua SS (2001) Inhibitory effect of acetosyringone on two aflatoxin biosynthetic genes. Lett Appl Microbiol 32:278
Hua SS, Grosjean OK, Baker JL (1999) Inhibition of aflatoxin biosynthesis by phenolic compounds. Lett Appl Microbiol 29:289
Iiyama K, Lam TB-T, Stone BA (1994) Covalent cross-links in the cell wall. Plant Physiol 104:315–320
Jaeck E, Dumas B, Geoffroy P, Favet N, Inze D, Van Montagu M, Fritig B, Legrand M (1992) Regulation of enzymes involved in lignin biosynthesis: induction of O-methyltransferase mRNAs during the hypersensitive reaction of tobacco to tobacco mosaic virus. Mol Plant Microbe Interact 5:294–300
Klinke HB, Ahring BK, Schmidt AS, Thomsen AB (2002) Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour Technol 82:15–26
Lee Y-W, Jin S, Sims W-S, Nester EW (1995) Genetic evidence for direct sensing of phenolic compounds by the VirA protein of Agrobacterium tumefaciens. Proc Natl Acad Sci USA 92:12245–12249
Lynn DG, Chen RH, Manning KS, Wood HN (1987) The structural characterization of endogenous factor from Vinca rosea crow, gall tumors that promote cell division of tobacco cells. Proc Natl Acad Sci USA 84:615–619
Martz F (1997) Modification de l’activité O-méthyltransférase dans des tabacs transgéniques: conséquences sur la lignine et la résistance au Virus de la Mosaïque du Tabac. PhD Thesis, Université Louis Pasteur, Strasbourg, France
Martz F, Maury S, Pinçon G, Legrand M (1998) cDNA cloning, substrate specificity and expression study of tobacco caffeoyl-CoA 3-O-methyltransferase, a lignin biosynthetic enzyme. Plant Mol Biol 36:427–437
Maury S (2000) Etude des O-méthyltransférases de la voie des phénylpropanoïdes chez le tabac et modulation de leur expression dans des tabacs transgéniques: conséquences sur la biosynthèse de la lignine et d’autres composés phénoliques et sur la résistance aux agents pathogènes. PhD Thesis, Université Louis Pasteur, Strasbourg, France
Maury S, Geoffroy P, Legrand M (1999) Tobacco O-methyltransferases involved in phenylpropanoid metabolism: the different CCoAOMT and COMT classes have distinct substrate specificities and expression patterns. Plant Physiol 121:215–224
McCullen CA, Binns AN (2006) Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu Rev Cell Dev Biol 22:101–127
Melchers LS, Regenburg-Tuïnk AJG, Schilperoort RA, Hooykaas PJ (1989) Specificity of signal molecules in the activation of Agrobacterium virulence gene expression. Mol Microbiol 3:969–977
Messens E, Dekeyser R, Stachel SE (1990) A nontransformable Triticum monococcum monocotyledonous culture produces the potent Agrobacterium vir-inducing compound ethyl ferulate. Proc Natl Acad Sci USA 87:4368–4372
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389
Orr JD, Lynn DG (1992) Biosynthesis of dehydrodiconiferyl alcohol glucosides: implications for the control of tobacco cell growth. Plant Physiol 98:343–352
Pellegrini LOG, Geoffroy P, Fritig B, Legrand M (1993) Molecular cloning and expression of a new class of ortho-diphenol-O-methyltransferases induced in tobacco leaves by infection or elicitor treatment. Plant Physiol 103:509–517
Pinçon G, Maury S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M (2001a) Repression of O-methyltransferase genes in transgenic tobacco affects lignin synthesis and plant growth. Phytochemistry 57:1167–1176
Pinçon G, Chabannes M, Lapierre C, Pollet B, Ruel K, Joseleau J-P, Boudet AM, Legrand M (2001b) Simultaneous down-regulation of caffeic/5-hydroxyferulic acid-O-methyltransferase I and cinnamoyl-coenzyme A reductase in the progeny from a cross between tobacco lines homozygous for each transgene consequences for plant development and lignin synthesis. Plant Physiol 2001:145–155
Shimoda N, Toyoda-Yamamoto A, Nugamine J, Usami S, Katayama M, Shakagami Y, Machida Y (1990) Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. Proc Natl Acad Sci USA 87:6684–6688
Stachel SE, Messens E, Van Montagu M, Zambryski P (1985a) Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629
Stachel SE, Gynheung A, Flores C, Nester EW (1985b) A Tn3 lacZ transposon for the random generation of β-galactosidase gene fusion; application to the analysis of gene expression in Agrobacterium. EMBO J 4:891–898
Tamagnone L, Merida A, Stacey N, Plaskitt K, Parr A, Chang C-F, Lynn D, Dow JM, Roberts K, Martin C (1998) Inhibition of phenolic acid metabolism results in precocious cell death and altered cell morphology in leaves of transgenic tobacco plants. Plant Cell 10:1801–1816
Teutonico RA, Dudley MW, Orr JD, Lynn DG, Binns AN (1991) Activity and accumulation of cell division-promoting phenolics in tobacco tissue cultures. Plant Physiol 97:288–297
Toquin V, Grausem B, Geoffroy P, Legrand M (2003) Structure of the tobacco caffeic acid O-methyltransferase (COMT) II gene: identification of promoter sequences involved in gene inductibility by various stimuli. Plant Mol Biol 52:495–509
Vance CP, Kirk TK, Sherwood RT (1980) Lignification as a mechanism of disease resistance. Annu Rev Phytopathol 18:259–288
Veena HJ, Doerge RW, Gelvin SB (2003) Transfer of T-DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defence gene expression. Plant J 35:219–236
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003a) Root exudation and rhizosphere biology. Plant Physiol 132:44–51
Walker TS, Bais HP, Halligan KM, Stermitz FR, Vivanco JM (2003b) Metabolic profiling of root exudates of Arabidopsis thaliana. J Agric Food Chem 51:2548–2554
Yuan Z-C, Edlind MP, Liu P, Saenkham P, Banta LM, Wise AA, Ronzone E, Binns AN, Kerr K, Nester EW (2007) The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci USA 104:11790–11795
Zhang J, Boone L, Kocz R, Zhang C, Binns AN, Lynn DG (2000) At the maize/Agrobacterium interface: natural factors limiting host transformation. Chem Biol 7:611–621
Zhong R, Morrison WH, Negrel J, Ye ZH (1998) Dual methylation pathway in lignin biosynthesis. Plant Cell 10:2033–2046
Zhu J, Oger PM, Schrammeijer B, Hooykaas PJJ, Farrand SK, Winans SC (2000) The bases of crown gall tumorigenesis. J Bacteriol 182:3885–3895
Acknowledgments
We are grateful to Professor F. Delmotte from LBLGC University of Orléans and Léon Otten from IBMP du CNRS of Strasbourg for providing Agrobacterium tumefaciens strains. We acknowledge Dr G. Pinçon, Dr F. Martz, Dr V. Toquin and Professor R. Atanassova for their previous works on OMT plants. We thank Professor E. Lainé and Professor Franck Brignolas from LBLGC for helpful discussions. We are grateful to A. Guichard for taking good care of tobacco plants and to Gilles Moreau France for technical assistance. This work was supported by the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2010_1230_MOESM1_ESM.pdf
FT-IR analysis of cell wall composition in transgenic stems after wounding or agroinfection. Average spectra were obtained from the cell wall analysis of eight replicates. C, control plants transformed with empty plasmid; COMTI, transgenic tobaccos suppressed for COMTI; COMTII, transgenic tobaccos suppressed for COMTII; CCoAOMT, transgenic tobaccos suppressed for CCoAOMT; dAS, transgenic tobaccos suppressed for COMTI and CCoAOMT. Unwounded (a), wounded and mock inoculated (b) or wounded and Agrobacterium tumefaciens infected transgenic plants (c) (Supplementary material 1 (PDF 74 kb)
425_2010_1230_MOESM2_ESM.pdf
Total soluble phenolic contents of transgenic stems after wounding or agroinfection. Total soluble phenolic compounds were extracted and quantified using Folin-Ciocalteu’s reagent. Extracts were prepared from 6-hours post-treatment tobacco stems of unwounded (white bars), wounded and mock inoculated (grey bars) or wounded and inoculated with a saturated culture of Agrobacterium tumefaciens (black bars) transgenic plants: C, control plants transformed with an empty plasmid; COMTI, transgenic tobaccos suppressed for COMTI; COMTII, transgenic tobaccos suppressed for COMTII; CCoAOMT, transgenic tobacco suppressed for CCoAOMT; dAS, transgenic tobacco suppressed for COMTI and CCoAOMT. Mean values ± SE (n=4) are shown. Two independent experiments were performed. In order to evaluate genotypic and treatment effects, two-way ANOVA analysis was performed: for each graph, g indicates the genotype effect, t the treatment effect and gxt the genotype by treatment interaction. Significant effects are indicated at * P ≤ 0.05 or ** P ≤ 0.01 (Supplementary material 2 (PDF 11 kb)
Rights and permissions
About this article
Cite this article
Maury, S., Delaunay, A., Mesnard, F. et al. O-methyltransferase(s)-suppressed plants produce lower amounts of phenolic vir inducers and are less susceptible to Agrobacterium tumefaciens infection. Planta 232, 975–986 (2010). https://doi.org/10.1007/s00425-010-1230-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1230-x