Abstract
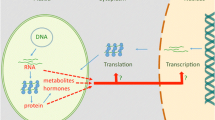
Genetic and physiological studies have to-date revealed evidence for five signaling pathways by which the chloroplast exerts retrograde control over nuclear genes. One of these pathways is dependent on product(s) of plastid protein synthesis, for another the signal is singlet oxygen, a third employs chloroplast-generated hydrogen peroxide, a fourth is controlled by the redox state of the photosynthetic electron transport chain, and a fifth involves intermediates and possibly proteins of tetrapyrrole biosynthesis. These five pathways may be part of a complex signaling network that links the functional and physiological state of the chloroplast to the nucleus. Mutants defective in various steps of photosynthesis reveal a surprising diversity in nuclear responses suggesting the existence of a complex signaling network.


Similar content being viewed by others
Abbreviations
- ALA:
-
5-aminolevulinic acid
- CHL H:
-
H subunit of Mg-chelatase
- CHL I:
-
I subunit of Mg-chelatase
- Chl:
-
Chlorophyll
- Chld:
-
Chlorophyllide
- DBMIB:
-
2,5-di-bromo-3-methyl-6-isopropyl-p-benzoquinone
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethyl urea
- gun :
-
Genome uncoupled
- LHC :
-
Gene for light-harvesting chlorophyll a/b binding protein
- MgProto:
-
Mg protoporphyrin IX
- MgProtoMe:
-
Mg protoporphyrin monomethyl ester
- Pchld:
-
Protochlorophyllide
- Proto:
-
Protoporphyrin IX
- Protogen:
-
Protoporphyrinogen
- PS:
-
Photosystem
- RBCS :
-
Gene for small subunit of ribulose bisphosphate carboxylase/oxygenase
- ROS:
-
Reactive oxygen species
References
Abdallah F, Salamini F, Leister D (2000) A prediction of the size and evolutionary origin of the proteome of chloroplasts of Arabidopsis. Trends Plant Sci 5:141–142
Adamska I (1995) Regulation of early light-inducible protein gene expression by blue and red light in etiolated seedlings involves nuclear and plastid factors. Plant Physiol 107:1167–1175
Ahlert D, Ruf S, Bock R (2003) Plastid protein synthesis is required for plant development in tobacco. Proc Natl Acad Sci USA 100:15730–15735
Alawady AE, Grimm B (2005) Tobacco Mg protoporphyrin IX methyltransferase is involved in inverse activation of Mg porphyrin and protoheme synthesis. Plant J 41:282–290
Anderson SL, Teakle GR, Martino-Catt SJ, Kay SA (1994) Circadian clock- and phytochrome-regulated transcription is conferred by a 78 bp cis-acting domain of the Arabidopsis CAB2 promoter. Plant J 6:457–470
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bajracharya D, Bergfield R, Hatzfeld W-D, Klein S, Schopfer P (1987) Regulatory involvement of plastids in the development of peroxisomal enzymes in the cotyledons of mustard (Sinapis alba L.) seedlings. J Plant Physiol 126:421–436
Beale SI (1999) Enzymes of chlorophyll biosynthesis. Photosynth Res 60:43–73
Beck CF (2001) Signalling pathways in chloroplast-to-nucleus communication. Protist 152:175–182
Beck CF, Grimm B (2005) Involvement of tetrapyrroles in cellular regulation. In: Grimm B, Porra RJ, Rüdiger W, Scheer H (eds) Biochemistry, biophysics and biological functions of Chlorophylls. Springer, The Netherlands (in press)
Boldt R, Börner T, Schnarrenberger C (1992) Repression of the plastidic isoenzymes of aldolase, 3-phosphoglycerate kinase, and triosephosphate isomerase in the barley mutant “albostrians”. Plant Physiol 99:895–900
Boldt R, Koshuchowa S, Gross W, Börner T, Schnarrenberger C (1997) Decrease in glycolate pathway enzyme activities in plastids and peroxisomes of the albostrians mutant of barley (Hordeum vulgare L.). Plant Sci 124:33–40
Bolle C, Kusnetsov VV, Herrmann RG, Oelmüller R (1996) The spinach AtpC and AtpD genes contain elements for light-regulated, plastid-dependent and organ-specific expression in the vicinity of the transcription start sites. Plant J 9:21–30
Börner T (1981) Enzymes of plastid ribosome-deficient mutants. Ferredoxin-NADP+ reductase. Biochem Physiol Pflanzen 176:737–743
Börner T (1986) Chloroplast control of nuclear gene function. Endocyt Cell Res 3:265–274
Börner T, Mendel RR, Schiemann J (1986) Nitrate reductase is not accumulated in chloroplast-ribosome-deficient mutants of higher plants. Planta 169:202–207
Bradbeer JW, Börner T (1978) Activities of glyceraldehyde-phosphate dehydrogenase (NADP+) and phosphoribulokinase in two barley mutants deficient in chloroplast ribosomes. In: Akoyunoglou G et al (eds) Chloroplast development. North-Holland Biomedical Press, Elsevier, pp 727–732
Bradbeer JW, Atkinson YE, Börner T, Hagemann R (1979) Cytoplasmic synthesis of plastid polypeptides may be controlled by plastid-synthesised RNA. Nature 279:816–817
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1158–1203
Chekounova E, Voronetskaja V, Papenbrock J, Grimm B, Beck CF (2001) Characterization of Chlamydomonas mutants defective in the H-subunit of Mg-chelatase. Mol Gen Genomics 266:363–373
Drazek ES, Hammack CA Sr, Schmitt MP (2000) Corynebacterium diphteriae genes required for acquisition of iron from haemin and haemoglobin are homologous to ABC haemin transporters. Mol Microbiol 36:68–84
Durnford DG, Falkowski PG (1997) Chloroplast redox regulation of nuclear gene transcription during photoacclimation. Photosynth Res 53:229–241
Emanuel C, Weihe A, Graner A, Hess WR, Börner T (2004) Chloroplast development affects expression of phage-type RNA polymerases in barley leaves. Plant J 38:460–472
Escoubas JM, Lomas M, La Roche J, Falkowski PG (1995) Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA 92:10237–10241
Fey V, Wagner R, Bräutigam K, Wirtz M, Hell R, Dietzmann A, Leister D, Oelmüller R, Pfannschmidt T (2005) Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J Biol Chem 280:5318–5328
Forsburg SL, Guarente L (1989) Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu Rev Cell Biol 5:153–180
Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33:691–705
Gibson LCD, Marrison JL, Leech RM, Jensen PE, Bassham DC, Gibson M, Hunter CN (1996) A putative Mg chelatase subunit from Arabidopsis thaliana cv C24. Plant Physiol 111:61–71
Goldschmidt-Clermont M (1998) Coordination of nuclear and chloroplast gene expression in plant cells. Int Rev Cytol 177:115–180
Gray JC (2003) Chloroplast-to-nucleus signalling: a role for Mg-protoporphyrin. Trends Genet 19:526–529
Gray JC, Sornarajah R, Zabron AA, Duckett CM, Khan MS (1995) Chloroplast control of nuclear gene expression. In: Mathis P (ed) Photosynthesis: from light to biosphere, vol III. Kluwer Acad Publishers, The Netherlands, pp 543–550
Gray JC, Sullivan JA, Wang JH, Jerome CA, MacLean D (2003) Coordination of plastid and nuclear gene expression. Philos Trans R Soc Lond B Biol Sci 358:135–144
Hagemann R, Börner T (1978) Plastid ribosome-deficient mutants of higher plants as a tool in studying chloroplast biogenesis. In: Akoyunoglou G et al (eds) Chloroplast development. North-Holland Biomedical Press, Elsevier, pp 709–720
Hammond-Kosack K, Jones JDG (2000) Responses to plant pathogens. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1102–1156
Hedtke B, Wagner I, Börner T, Hess WR (1999) Interorganellar crosstalk in higher plants: impaired chloroplast development affects mitochondrial gene and transcript levels. Plant J 19:635–644
Hess WR, Schendel R, Börner T, Rüdiger W (1991) Reduction of mRNA level for two nuclear encoded light regulated genes in the barley mutant albostrians is not correlated with phytochrome content and activity. J Plant Physiol 138:292–298
Hess WR, Müller A, Nagy F, Börner T (1994) Ribosome-deficient plastids affect transcription of light-induced nuclear genes: genetic evidence for a plastid-derived signal. Mol Gen Genet 242:305–312
Hon T, Hach A, Tamalis D, Zhu Y, Zhang L (1999) The yeast heme-responsive transcriptional activator Hap1 is a preexisting dimer in the absence of heme. J Biol Chem 274:22770–22774
Ihnatowicz A, Pesaresi P, Varotto C, Richly E, Schneider A, Jahns P, Salamini F, Leister D (2004) Mutants for photosystem I subunit D of Arabidopsis thaliana: effects on photosynthesis, photosystem I stability and expression of nuclear genes for chloroplast functions. Plant J 37:839–852
Jacobs JM, Jacobs NJ (1993) Porphyrin accumulation and export by isolated barley (Hordeum vulgare) plastids. Plant Physiol 101:1181–1187
Johanningmeier U (1988) Possible control of transcript levels by chlorophyll precursors in Chlamydomonas. Eur J Biochem 177:417–424
Johanningmeier U, Howell SH (1984) Regulation of light-harvesting chlorophyll-binding protein mRNA accumulation in Chlamydomonas reinhardtii. J Biol Chem 259:13541–13549
Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9:627–640
Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284:654–657
Kimura M, Manabe K, Abe T, Yoshida S, Matsui M, Yamamoto YY (2003) Analysis of hydrogen peroxide-independent expression of the high-light-inducible ELIP2 gene with the aid of the ELIP2 promoter-luciferase fusion. Photochem Photobiol 77:668–674
Krieger-Liszkay A (2004) Singlet oxygen production in photosynthesis. J Exp Bot 56:337–346
Kropat J, Beck CF (1998) Characterization of photoreceptor and signalling pathway for light induction of the Chlamydomonas heat-shock gene HSP70A. Photochem Photobiol 68:414–419
Kropat J, Oster U, Rüdiger W, Beck CF (1997) Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc Natl Acad Sci USA 94:14168–14172
Kropat J, Pöpperl G, Rüdiger W, Beck CF (1999) Identification of Mg-protoporphyrin IX as a chloroplast signal that mediates the expression of nuclear genes. In: Wagner E, Normann J, Greppin H, Hackstein JHP, Hermann RG, Kowallik KV, Schenk HEA, Seckbach J (eds) From symbiosis to eukaryotism. Endocytobiol VII. University of Geneva, Geneva, pp 341–348
Kropat J, Oster U, Rüdiger W, Beck CF (2000) Chloroplast signalling in the light induction of nuclear HSP70 genes requires the accumulation of chlorophyll precursors and their accessibility to cytoplasm/nucleus. Plant J 24:523–531
Kusnetsov V, Bolle C, Lubberstedt T, Sopory S, Herrmann RG, Oelmüller R (1996) Evidence that the plastid signal and light operate via the same cis-acting elements in the promoters of nuclear genes for plastid proteins. Mol Gen Genet 252:631–639
La Rocca N, Rascio N, Oster U, Rüdiger W (2001) Amitrole treatment of etiolated barley seedlings leads to deregulation of tetrapyrrole synthesis and to reduced expression of Lhc and RbcS genes. Planta 213:101–108
Larkin RM, Alonso JM, Ecker JR, Chory J (2003) GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299:902–906
Leegood RC, Walker DA, Foyer CH (1985) Regulation of the Benson-Calvin cycle. In: Barber J, Baker NR (eds) Photosynthetic mechanisms and the environment. Elsevier Science Publishers, Amsterdam, pp 199–258
Leon P, Arroyo A, Mackenzie S (1998) Nuclear control of plastid and mitochondrial development in higher plants. Annu Rev Plant Physiol Plant Mol Biol 49:453–480
Lindemann P, Koch A, Degenhardt B, Hause G, Grimm B, Papadopoulos V (2004) A novel Arabidopsis thaliana protein is a functional peripheral-type benzodiazepine receptor. Plant Cell Physiol 45:723–733
Møller SG, Kunkel T, Chua N-H (2001) A plastidic ABC protein involved in intercompartmental communication of light signalling. Genes Dev 15:90–103
Maiwald D, Dietzmann A, Jahns P, Pesaresi P, Joliot P, Joliot A, Levin JZ, Salamini F, Leister D (2003) Knock-out of the genes coding for the Rieske protein and the ATP-synthase δ-subunit of Arabidopsis. Effects on photosynthesis, thylakoid protein composition, and nuclear chloroplast gene expression. Plant Physiol 133:191–202
Martin W, Rujan T, Richly E, Hansen A, Cornelsen S, Lins T, Leister D, Stoebe B, Hasegawa M, Penny D (2002) Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc Natl Acad Sci USA 99:12246–12251
Martínez-Hernández A, López-Ochoa L, Argüello-Astorga G, Herrera-Estrella L (2002) Functional properties and regulatory complexity of a minimal RCBS light-responsive unit activated by phytochrome, cryptochrome, and plastid signals. Plant Physiol 128:1223–1233
Maul JE, Lilly JW, Cui L, dePamphilis CW, Miller W, Harris EH, Stern DB (2002) The Chlamydomonas reinhardtii plastid chromosome: islands of genes in a sea of repeats. Plant Cell 14:2659–2679
Maxwell DP, Laudenbach DE, Huner NPA (1995) Redox regulation of light-harvesting complex II and cab mRNA abundance in Dunaliella salina. Plant Physiol 109:787–795
McCormac AC, Terry MJ (2004) The nuclear genes Lhcb and HEMA1 are differentially sensitive to plastid signals and suggest distinct roles for the GUN1 and GUN5 plastid-signalling pathways during de-etiolation. Plant J 40:672–685
Meskauskiene R, Apel K (2002) Interaction of FLU, a negative regulator of tetrapyrrole biosynthesis, with the glutamyl-tRNA reductase requires the tetratricopeptide repeat domain of FLU. FEBS Lett 532:27–30
Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K (2001). FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 98:12826–12831
Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J (2001) Arabidopsis genomes uncoupled 5 (GUN 5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98:2053–2058
Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5:43–48
Nakayama M, Masuda T, Bando T, Yamagata H, Ohta H, Takamiya K (1998) Cloning and expression of the soybean chlH gene encoding a subunit of Mg-Chelatase and localization of the Mg2+ concentration-dependent ChlH protein within the chloroplast. Plant Cell Physiol 39:275–284
Oelmüller R (1989) Photooxidative destruction of chloroplasts and its effect on nuclear gene expression and extraplastidic enzyme levels. Photochem Photobiol 49:229–239
Oelmüller R, Mohr H (1986a) Photooxidative destruction or chloroplasts and its consequences for expression of nuclear genes. Planta 167:106–113
Oelmüller R, Levitan I, Bergfeld R, Rajasekhar VK, Mohr H (1986b) Expression of nuclear genes is affected by treatments acting on plastids. Planta 168:482–492
Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibehara S, Fujita H, Igarashi K (2001) Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J 20:2835–2843
op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, Nater M, Apel K (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15:2320–2332
Oster U, Brunner H, Rüdiger W (1996) The greening process in cress seedlings. V. Possible interference of chlorophyll precursors, accumulated after thujaplicin treatment, with light-regulated expression of Lhc genes. J Photochem Photobiol B 36:255–261
Papenbrock J, Grimm B (2001) Regulatory network of tetrapyrrole biosynthesis—studies for intracellular signalling involved in metabolic and developmental control of plastids. Planta 213:667–681
Papenbrock J, Mock H-P, Tanaka R, Kruse E, Grimm B (2000a) Role of magnesium chelatase activity in the early steps of the tetrapyrrole biosynthetic pathway. Plant Physiol 122:1161–1169
Papenbrock J, Pfündel E, Mock H-P, Grimm B (2000b) Decreased and increased expression of the subunit CHL I diminishes Mg-chelatase activity and reduces chlorophyll synthesis in transgenic tobacco plants. Plant J 22:155–164
Petracek ME, Dickey LF, Thompson WF (1997) Light-regulated changes in abundance and polyribosome association of ferrodoxin are dependent on photosynthesis. Plant Cell 9:2291–2300
Petracek ME, Dickey LF, Nguyen TT, Gatz G, Sowinski DA, Allen GC, Thompson WF (1998) Ferredoxin-1 mRNA is destabilized by changes in photosynthetic electron transport. Proc Natl Acad Sci USA 95:9009–9013
Pfannschmidt T (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci 1:33–41
Pfannschmidt T, Nilsson A, Allen JF (1999) Photosynthetic control of chloroplast gene expression. Nature 397:625–628
Pfannschmidt T, Schütz K, Brost M, Oelmüller R (2001) A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J Biol Chem 276:36125–36130
Pfannschmidt T, Schütz K, Fey V, Sherameti I, Oelmüller R (2003) Chloroplast redox control of nuclear gene expression—a new class of plastid signals in interorganellar communication. Antiox Redox Signal 5:95–101
Pursiheimo S, Mulo P, Rintamäki E, Aro E-M (2001) Coregulation of light-harvesting complex II phosphorylation and Lhcb accumulation in winter rye. Plant J 26:317–327
Reiss T, Bergfeld R, Link G, Thien W, Mohr H (1983) Photooxidative destruction of chloroplasts and its consequences to cytosolic enzyme levels and plant development. Planta 159:518–528
Richly E, Dietzmann A, Biehl A, Kurth J, Laloi C, Apel K, Salamini F, Leister D (2003) Covariations in the nuclear chloroplast transcriptome reveal a regulatory master-switch. EMBO reports 4:491–498
Rodermel S (2001) Pathway of plastid-to-nucleus signalling. Trends Plant Sci 6:471–478
Rujan T, Martin W (2001) How many genes in Arabidopsis came from cyanobacteria? An estimate from 386 protein phylogenies. Trends Genet 17:113–120
Schroda M, Vallon O, Wollman F-A, Beck CF (1999) A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell 11:1165–1178
Sherameti I, Sopory SK, Trebicka A, Pfannschmidt T, Oelmüller R (2002) Photosynthetic electron transport determines nitrate reductase gene expression and activity in higher plants. J Biol Chem 277:46594–46600
Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O (2004) Iron-source preference of staphylococcus aureus infections. Science 305:1626–1628
Stojiljkovic I, Hantke K (1994) Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol 13:719–732
Strand A (2004) Plastid-to-nucleus signalling. Curr Opin Plant Biol 7:621–625
Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421:79–83
Sullivan JA, Gray JC (1999) Plastid translation is required for the expression of nuclear photosynthesis genes in the dark and in roots of the pea lip1 mutant. Plant Cell 11:901–910
Sullivan JA, Gray JC (2002) Multiple plastid signals regulate the expression of the pea plastocyanin gene in pea and transgenic tobacco plants. Plant J 32:763–774
Surpin M, Larkin RM, Chory J (2002) Signal transduction between the chloroplast and the nucleus. Plant Cell 14 (Suppl):S327–S338
Susek RE, Ausubel FM, Chory J (1993) Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74:787–799
Taylor WC (1989) Regulatory interactions between nuclear and plastid genomes. Annu Rev Plant Physiol Plant Mol Biol 40:211–233
Terry MJ, Kendrick RE (1999) Feedback inhibition of chlorophylI synthesis in the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato. Plant Physiol 119:143–152
Terzaghi WB, Cashmore AR (1995) Light-regulated transcription. Annu Rev Plant Physiol Plant Mol Biol 46:445–474
Thomas J, Weinstein JD (1990) Measurement of heme efflux and heme content in isolated developing chloroplasts. Plant Physiol 94:1414–1423
Vasileuskaya Z, Oster U, Beck CF (2004) Involvement of tetrapyrroles in inter-organellar signaling in plants and algae. Photosynth Res 82:289–299
Vasileuskaya Z, Oster U, Beck CF (2005) Identification of Mg-protoporphyrinIX and heme as plastid/mitochondrial factors that control HEMA expression in Chlamydomonas. Eukaryotic Cell 4 (in press)
Vothknecht UC, Kannangara CG, von Wettstein D (1996) Expression of catalytically active barley glutamyl tRNAGlu reductase in Escherichia coli as a fusion protein with glutathione S-transferase. Proc Natl Acad Sci USA 93:9287–9291
Wagner D, Przybyla D, op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, Apel K (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306:1183–1185
Walker CJ, Willows RD (1997) Mechanism and regulation of Mg-chelatase. Biochem J 327:321–333
Weinstein JD, Beale SI (1985) Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys 237:454–464
Yabuta Y, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S (2004) Two distinct redox signaling pathways for cytosolic APX induction under photooxidative stress. Plant Cell Physiol 45:1586–1594
Acknowledgements
The author wishes to thank Bernhard Grimm, Wolfgang Hess, Anja Liszkay-Krieger, Thomas Pfannschmidt and Michael Schroda for constructive comments on the manuscript. He also wishes to thank Ning Shao and Zinaida Vasileuskaya for drawing of the figures. This project was supported by a grant of the DFG (BE903/13-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beck, C.F. Signaling pathways from the chloroplast to the nucleus. Planta 222, 743–756 (2005). https://doi.org/10.1007/s00425-005-0021-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0021-2