Abstract
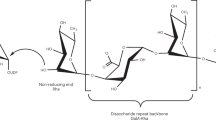
We investigated the properties of a galactosyltransferase (GalT) that is involved in the synthesis of β-(1→4)-galactan side chains of pectins. A membrane preparation of etiolated 6-day-old soybean (Glycine max Merr.) hypocotyls transferred [14C]Gal from UDP-[14C]Gal into intact and partially hydrolyzed lupin β-(1→4)-galactans of various chain lengths as exogenous acceptors, while activity to endogenous acceptors was negligible. Maximal activity occurred at pH 6.5 and 20–25°C in the presence of 25 mM Mn2+ and 0.75% Triton X-100. The transfer reaction onto the unmodified commercial pectic galactan (M r>150,000) from lupin we used was very low but increased when the M r of the galactan was reduced by partial acid hydrolysis. Among the partially hydrolyzed galactans, high-M r (average M r 60,000) β-(1→4)-galactan was a more efficient acceptor [specific activity 2,000–3,000 pmol min−1 (mg protein)−1] than low-M r (average M r 10,000 and 5,000) polymers. Digestion of the radiolabeled product from high-M r galactan with endo-β-(1→4)-galactanase released mainly radioactive β-(1→4)-galactobiose and Gal, indicating that the transfer of [14C]Gal occurred through β-(1→4)-linkages. HPLC analysis showed that the enzyme also catalyzes incorporation of Gal into pyridylaminated (PA) β-(1→4)-galactooligomers with degree of polymerization at least 5. Gal7-PA chains were elongated by attachment of one, two, or three Gal residues leading to the formation of Gal8–10-PA.







Similar content being viewed by others
Abbreviations
- AGP :
-
Arabinogalactan-protein
- Ara :
-
Arabinose
- DP :
-
Degree of polymerization
- GalA :
-
Galacturonic acid
- Gal n -PA :
-
Pyridylaminated β-(1→4)-galactooligosaccharides
- GalT :
-
Galactosyltransferase
- MALDI–TOF–MS :
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- Rha :
-
Rhamnose
References
Akita K, Ishimizu T, Tsukamoto T, Ando T, Hase S (2002) Successive glycosyltransfer activity and enzymatic characterization of pectic polygalacturonate 4-α-galacturonosyltransferase solubilized from pollen tubes of Petunia axillaris using pyridylaminated oligogalacturonates as substrates. Plant Physiol 130:374–379
Albersheim P, Nevins DJ, English PD, Karr A (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas liquid chromatography. Carbohydr Res 5:340–345
Abdel-Massih RM, Baydoun EAH, Brett CT (2003) In vitro biosynthesis of 1,4-β-galactan attached to a pectin–xyloglucan complex in pea. Planta 216:502–511
Al-Kaisey MT, Wilkie KCB (1992) The polysaccharides of agricultural lupin seeds. Carbohydr Res 227:147–161
Baydoun EAH, Abdel-Massih RM, Dani D, Rizk SE, Brett CT (2001) Galactosyl- and fucosyltransferases in etiolated pea epicotyls: product identification and sub-cellular localisation. J Plant Physiol 158:145–150
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brickell LS, Reid JSG (1996) Biosynthesis in vitro of pectic (1→4)-β-d-galactan. In: Visser J, Voragen AGJ (eds) Pectins and pectinases. Elsevier, Amsterdam, pp 127–134
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ermel FF, Follet-Gueye ML, Cibert C, Vian B, Morvan C, Catesson AM, Goldberg R (2000) Differential localization of arabinan and galactan side chains of rhamnogalacturonan 1 in cambial derivatives. Planta 210:732–740
Geshi N, Jørgensen B, Scheller HV, Ulvskov P (2000) In vitro biosynthesis of 1,4-β-galactan attached to rhamnogalacturonan I. Planta 210:622–629
Geshi N, Pauly M, Ulvskov P (2002) Solubilization of galactosyltransferase that synthesizes 1,4-β-galactan side chains in pectic rhamnogalacturonan I. Physiol Plant 114:540–548
Goubet F, Morvan C (1993) Evidence for several galactan synthases in flax (Linum usitatissimum L.) suspension-cultured cells. Plant Cell Physiol 34:1297–1303
Hakomori S (1964) A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J Biochem 55:205–208
Ishikawa M, Kuroyama H, Takeuchi Y, Tsumuraya Y (2000) Characterization of pectin methyltransferase from soybean hypocotyls. Planta 210:782–791
Iwai H, Ishii T, Satoh S (2001) Absence of arabinan in the side chains of the pectic polysaccharides strongly associated with cell walls of Nicotiana plumbaginifolia non-organogenic callus with loosely attached constituent cells. Planta 213:907–915
Kato H, Takeuchi Y, Tsumuraya Y, Hashimoto Y, Nakano H, Kováč P (2003) In vitro biosynthesis of galactans by membrane-bound galactosyltransferase from radish (Raphanus sativus L.) seedlings. Planta 217:271–282
Kobayashi M, Matoh T, Azuma J (1996) Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plants cell walls. Plant Physiol 110:1017–1020
Kondo A, Suzuki J, Kuraya N, Hase S, Kato I, Ikenaka T (1990) Improved method for fluorescence labeling of sugar chains with sialic acid residues. Agric Biol Chem 54:2169–2170
Kuroyama H, Tsumuraya Y (2001) A xylosyltransferase that synthesizes β-(1→4)-xylans in wheat (Triticum aestivum L.) seedlings. Planta 213:231–240
McCartney L, Ormerod AP, Gidley MJ, Knox JP (2000) Temporal and spatial regulation of pectic (1→4)-β-d-galactan in cell walls of developing pea cotyledons: implications for mechanical properties. Plant J 22:105–113
Misawa H, Tsumuraya Y, Kaneko Y, Hashimoto Y (1996) α-l-Fucosyltransferases from radish primary roots. Plant Physiol 110:665–673
Nakamura A, Furuta H, Maeda H, Takao T, Nagamatsu Y (2002) Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Biosci Biotechnol Biochem 66:1301–1313
Nakano H, Takenishi S, Watanabe Y (1985) Purification and properties of two galactanases from Penicillium citrinum. Agric Biol Chem 49:3445–3454
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Okemoto K, Uekita T, Tsumuyara Y, Hashimoto Y, Kasama T (2003) Purification and characterization of an endo-β-(1→6)-galactanase from Trichoderma viride. Carbohydr Res 338:219–230
O’Neill M, Albersheim P, Darvill A (1990) The pectic polysaccharides of primary cell walls. In: Dey PM (ed) Methods in plant biochemistry, vol 2. Academic Press, London, pp 415–491
Panayotatos N, Villemez CL (1973) The formation of a β-(1→4)-d-galactan chain catalysed by a Phaseolus aureus enzyme. Biochem J 133:263–271
Peugnet I, Goubet F, Bruyant-Vannier MP, Thoiron B, Morvan C, Schols HA, Voragen AGJ (2001) Solubilization of rhamnogalacturonan I galactosyltransferases from membranes of a flax cell suspension. Planta 213:435–445
Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967
Saxena IM, Brown RM Jr, Fevre M, Geremia RA, Henrissat B (1995) Multidomain architecture of β-glycosyl transferases: implications for mechanism of action. J Bacteriol 177:1419–1424
Sekimata M, Ogura K, Tsumuraya Y, Hashimoto Y, Yamamoto S (1989) A β-galactosidase from radish (Raphanus sativus L.) seedlings. Plant Physiol 90:567–574
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Sørensen SO, Pauly M, Bush M, Skjøt M, McCann MC, Borkhardt B, Ulvskov P (2000) Pectin engineering: modification of potato pectin by in vivo expression of an endo-1,4-β-d-galactanase. Proc Natl Acad Sci USA 97:7639–7644
Tsumuraya Y, Mochizuki N, Hashimoto Y, Kováč P (1990) Purification of an exo-β-(1→3)-d-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabinogalactan-proteins. J Biol Chem 265:7207–7215
Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells. Plant Physiol 99:1070–1083
Acknowledgements
We thank Dr. K. Hori of the Akita Research Institute of Food & Brewing, Akita, Japan, for carrying out the GC/MS spectrometry. We also thank Dr. H. Nakano, Osaka Municipal Research Institute, for the gift of endo-β-(1→4)-galactanase. This study was supported in part by a grant-in-aid (Glyco-technology Project) from the Ministry of Agriculture, Forestry, and Fisheries, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sugars described in this paper belong to the d-series unless otherwise noted
Rights and permissions
About this article
Cite this article
Konishi, T., Mitome, T., Hatsushika, H. et al. Biosynthesis of pectic galactan by membrane-bound galactosyltransferase from soybean (Glycine max Merr.) seedlings. Planta 218, 833–842 (2004). https://doi.org/10.1007/s00425-003-1163-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1163-8