Abstract
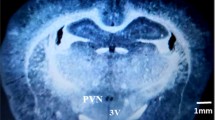
Chemerin is an adipocytokine involved in inflammation and lipid metabolism via G protein-coupled receptor, chemokine-like receptor (CMKLR)1. Since the important nuclei regulating pressure (BP) exist in the brain, we examined the effects of acute intracerebroventricular (i.c.v.) injection of chemerin-9 on systemic BP and explored underlying mechanisms. We examined the effects of acute i.c.v. injection of chemerin-9 (10 nmol/head) on systemic BP by a carotid cannulation method in the control or CMKLR1 small interfering (si) RNA-treated Wistar rats (0.04 nmol, 3 days, i.c.v.). We examined protein expression of CMKLR1 around brain ventricles by Western blotting. We examined the effects of acute i.c.v. injection of chemerin-9 on serum adrenaline by a high performance liquid chromatography. In the control siRNA-treated rats, chemerin-9 significantly increased mean BP, which reached a peak at 2 to 4 min after injection. On the other hand, in the CMKLR1 siRNA-treated rats, chemerin-9 did not affect the mean BP. Protein expression of CMKLR1 specifically in subfornical organ (SFO) and paraventricular nucleus (PVN) from the CMKLR1 siRNA-treated rats decreased compared with the control siRNA-treated rats. In the control siRNA-treated rats, chemerin-9 increased serum adrenaline level. On the other hand, in the CMKLR1 siRNA-treated rats, chemerin-9 did not affect the serum adrenaline level. Further, pretreatment with prazosin, an α-adrenaline receptor blocker, significantly prevented the pressor responses induced by chemerin-9. In summary, we for the first time demonstrated that chemerin-9 stimulates the sympathetic nerves via CMKLR1 perhaps expressed in SFO and PVN, which leads to an increase in systemic BP.





Similar content being viewed by others
References
Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D (2007) Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148:4687–4694. https://doi.org/10.1210/en.2007-0175
Brunetti L, Di Nisio C, Recinella L, Chiavaroli A, Leone S, Ferrante C, Orlando G, Vacca M (2011) Effects of vaspin, chemerin and omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptides 32:1866–1871. https://doi.org/10.1016/j.peptides.2011.08.003
Buechler C, Feder S, Haberl EM, Aslanidis C (2019) Chemerin isoforms and activity in obesity. Int J Mol Sci 20:1–16. https://doi.org/10.3390/ijms20051128
Caron A, Lee S, Elmquist JKGL (2016) Leptin and brain–adipose crosstalks Alexandre. Physiol Behav 176:139–148. https://doi.org/10.1016/j.physbeh.2017.03.040
Chen S, Gouaux E (2019) Structure and mechanism of AMPA receptor — auxiliary protein complexes. Curr Opin Struct Biol 54:104–111. https://doi.org/10.1016/j.sbi.2019.01.011
Chiu C, Miller MC, Caralopoulos IN, Worden MS, Brinker T, Gordon ZN, Johanson CE, Silverberg GD (2012) Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS 9:1–8. https://doi.org/10.1186/2045-8118-9-3
Darios ES, Winner BM, Charvat T, Krasinksi A, Punna S, Watts SW (2016) The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am J Physiol Heart Circ Physiol 311:H498–H507. https://doi.org/10.1152/ajpheart.00998.2015
El-Werfali W, Toomasian C, Maliszewska-Scislo M, Li C, Rossi NF (2015) Haemodynamic and renal sympathetic responses to V1b vasopressin receptor activation within the paraventricular nucleus. Exp Physiol 100:553–565. https://doi.org/10.1113/expphysiol.2014.084426
Ferland DJ, Darios ES, Neubig RR, Sjögren B, Truong N, Torres R, Dexheimer TS, Thompson JM, Watts SW (2018) Chemerin-induced arterial contraction is Gi - and calcium- dependent. 88:30–41. https://doi.org/10.1016/j.vph.2016.11.009.Chemerin-induced
Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ (2007) Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282:28175–28188. https://doi.org/10.1074/jbc.M700793200
Guo X, Fu Y, Xu Y, Weng S, Liu D, Cui D, Yu S, Liu X, Jiang K, Dong Y (2012) Chronic mild restraint stress rats decreased CMKLR1 expression in distinct brain region. Neurosci Lett 524:25–29. https://doi.org/10.1016/j.neulet.2012.06.075
Hallbeck M, Blomqvist A (1999) Spinal cord-projecting vasopressinergic neurons in the rat paraventricular hypothalamus. J Comp Neurol 411:201–211. https://doi.org/10.1002/(SICI)1096-9861(19990823)411:2<201::AID-CNE3>3.0.CO;2-3
Harland D, Gardiner SM, Bennett T (1989) Differential cardiovascular effects of centrally administered vasopressin in conscious Long Evans and Brattleboro rats. Circ Res 65:925–933. https://doi.org/10.1161/01.RES.65.4.925
Helfer G, Ross AW, Thomson LM, Mayer CD, Stoney PN, McCaffery PJ, Morgan PJ (2016) A neuroendocrine role for chemerin in hypothalamic remodelling and photoperiodic control of energy balance. Sci Rep 6:1–12. https://doi.org/10.1038/srep26830
Helfer G, Wu QF (2018) Chemerin: a multifaceted adipokine involved in metabolic disorders. J Endocrinol 238:R79–R94. https://doi.org/10.1530/JOE-18-0174
De Henau O, Degroot GN, Imbault V, Robert V, De Poorter C, McHeik S, Galés C, Parmentier M, Springael JY (2016) Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PLoS One 11:1–20. https://doi.org/10.1371/journal.pone.0164179
Hernandes MS, Troncone LRP (2009) Glycine as a neurotransmitter in the forebrain: a short review. J Neural Transm 116:1551–1560. https://doi.org/10.1007/s00702-009-0326-6
Hirooka Y, Sunagawa K (2010) Hypertension, heart failure, and the sympathetic nervous system: from the regulatory abvormality as the pathophysiology to novel therapeutic aspects. Fukuoka Igaku Zasshi 101:190–197
Hiyama TY, Noda M (2016) Sodium sensing in the subfornical organ and body-fluid homeostasis. Neurosci Res 113:1–11. https://doi.org/10.1016/j.neures.2016.07.007
Huber G, Schuster F, Raasch W (2017) Brain renin-angiotensin system in the pathophysiology of cardiovascular diseases. Pharmacol Res 125:72–90. https://doi.org/10.1016/j.phrs.2017.06.016
Imoto K, Hirakawa M, Okada M, Yamawaki H (2018) Canstatin modulates L-type calcium channel activity in rat ventricular cardiomyocytes. Biochem Biophys Res Commun 499:954–959. https://doi.org/10.1016/j.bbrc.2018.04.026
Kaur J, Adya R, Tan BK, Chen J, Randeva HS (2010) Identification of chemerin receptor (ChemR23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun 391:1762–1768. https://doi.org/10.1016/j.bbrc.2009.12.150
Kc P, Dick T (2010) Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Respir Physiol Neurobiol 174:55–64. https://doi.org/10.1038/jid.2014.371
Kennedy AJ, Davenport AP (2018) International union of basic and clinical pharmacology CIII: Chemerin receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) nomenclature, pharmacology, and function. Pharmacol Rev 70:174–196. https://doi.org/10.1124/pr.116.013177
Kodama T, Okada M, Yamawaki H (2019) Eukaryotic elongation factor 2 kinase inhibitor, A484954 inhibits noradrenaline-induced acute increase of blood pressure in rats. J Vet Med Sci 81:35–41. https://doi.org/10.1292/jvms.18-0606
Kostopoulos CG, Spiroglou SG, Varakis JN, Apostolakis E, Papadaki HH (2014) Chemerin and CMKLR1 expression in human arteries and periadventitial fat: a possible role for local chemerin in atherosclerosis? BMC Cardiovasc Disord 14:1–9. https://doi.org/10.1186/1471-2261-14-56
Kubo T (2006) Mechanisms of hypertension in the central nervous system. Yakugaku Zasshi 126:695–709. https://doi.org/10.1248/yakushi.126.695
Kunimoto H, Kazama K, Takai M, Oda M, Okada M, Yamawaki H (2015) Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am J Physiol Heart Circ Physiol 309:H1017–H1028. https://doi.org/10.1152/ajpheart.00820.2014
Lee B, Shao J (2014) Adiponectin and energy homeostasis. Rev Endocr Metab Disord 15:149–156. https://doi.org/10.1007/s11154-013-9283-3
Lozić M, Šarenac O, Murphy D, Japundžić-Žigon N (2018) Vasopressin, central autonomic control and blood pressure regulation. Curr Hypertens Rep 20:1–7. https://doi.org/10.1007/s11906-018-0811-0
Malinow R (2003) AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci 358:707–714. https://doi.org/10.1098/rstb.2002.1233
Nomura K, Hiyama TY, Sakuta H, Matsuda T, Lin CH, Kobayashi K, Kobayashi K, Kuwaki T, Takahashi K, Matsui S, Noda M (2019) [Na + ] increases in body fluids sensed by central Na x induce sympathetically mediated blood pressure elevations via H + −dependent activation of ASIC1a. Neuron 101:60–75.e6. https://doi.org/10.1016/j.neuron.2018.11.017
Okada S, Murakami Y, Nakamura K, Yokotani K (2002) Vasopressin V1 receptor-mediated activation of central sympatho-adrenomedullary outflow in rats. Eur J Pharmacol 457:29–35. https://doi.org/10.1016/S0014-2999(02)02652-3
Okada S, Yamaguchi N (2010) α1-Adrenoceptor activation is involved in the central N-methyl-d-aspartate-induced adrenomedullary outflow in rats. Eur J Pharmacol 640:55–62. https://doi.org/10.1016/j.ejphar.2010.04.038
Otani K, Okada M, Yamawaki H (2017) Diverse distribution of tyrosine receptor kinase b isoforms in rat multiple tissues. J Vet Med Sci 79:1516–1523. https://doi.org/10.1292/jvms.17-0257
Paxinos G, Watson C (2014) The rat brain in stereotaxic coordinates: hard cover edition. Elsevier
Rhea EM, Salameh TS, Logsdon AF, Hanson AJ, Erickson MA, Banks WA (2017) Blood-brain barriers in obesity. AAPS J 19:921–930. https://doi.org/10.1208/s12248-017-0079-3
Gun RS, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sichi S (2007) Chemerin-a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun 362:1013–1018. https://doi.org/10.1016/j.bbrc.2007.08.104
Rourke JL, Dranse HJ, Sinal CJ (2013) Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes Rev 14:245–262. https://doi.org/10.1111/obr.12009
Rourke JL, Muruganandan S, Dranse HJ, McMullen NM, Sinal CJ (2014) Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J Endocrinol 222:201–215. https://doi.org/10.1530/JOE-14-0069
Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clément K (2010) Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 95:2892–2896. https://doi.org/10.1210/jc.2009-2374
Shimamura K, Matsuda M, Miyamoto Y, Yoshimoto R, Seo T, Tokita S (2009) Identification of a stable chemerin analog with potent activity toward ChemR23. Peptides 30:1529–1538. https://doi.org/10.1016/j.peptides.2009.05.030
Shimizu T, Shimizu S, Higashi Y, Nakamura K, Yoshimura N, Saito M (2016) A stress-related peptide bombesin centrally induces frequent urination through brain bombesin receptor types 1 and 2 in the rat. J Pharmacol Exp Ther 356:693–701. https://doi.org/10.1124/jpet.115.230334
Weng C, Shen Z, Li X, Jiang W, Peng L, Yuan H, Yang K, Wang J (2017) Effects of chemerin/CMKLR1 in obesity-induced hypertension and potential mechanism. Am J Transl Res 9:3096–3104
Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D (2003) Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 198:977–985. https://doi.org/10.1084/jem.20030382
Wittamer V, Grégoire F, Robberecht P, Vassart G, Communi D, Parmentier M (2004) The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem 279:9956–9962. https://doi.org/10.1074/jbc.M313016200
Yamawaki H (2011) Vascular effects of novel adipocytokines: focus on vascular contractility and inflammatory responses. Biol Pharm Bull 34:307–310. https://doi.org/10.1248/bpb.34.307
Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC (2005) Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem 280:34661–34666. https://doi.org/10.1074/jbc.M504868200
Zhang Y, Xu N, Ding Y, Zhang Y, Li Q, Flores J, Haghighiabyaneh M, Doycheva D, Tang J, Zhang JH (2018) Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav Immun 70:179–193. https://doi.org/10.1016/j.bbi.2018.02.015
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number 17H03918 and Kitasato University Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1055 kb)
Rights and permissions
About this article
Cite this article
Yamamoto, A., Matsumoto, K., Hori, K. et al. Acute intracerebroventricular injection of chemerin-9 increases systemic blood pressure through activating sympathetic nerves via CMKLR1 in brain. Pflugers Arch - Eur J Physiol 472, 673–681 (2020). https://doi.org/10.1007/s00424-020-02391-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02391-4