Abstract
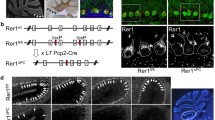
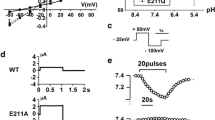
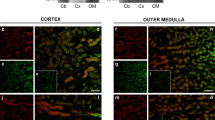
SLC26A11 (human)/Slc26a11 (mouse), also known as kidney brain anion transporter (KBAT), is a member of the SLC26 anion transporter family and shows abundant mRNA expression in the brain. However, its exact cellular distribution and subcellular localization in the brain and its functional identity and possible physiological roles remain unknown. Expression and immunostaining studies demonstrated that Slc26a11 is abundantly expressed in the cerebellum, with a predominant expression in Purkinje cells. Lower expression levels were detected in hippocampus, olfactory bulb, cerebral cortex, and subcortical structures. Patch clamp studies in HEK293 cells transfected with mouse cDNA demonstrated that Slc26a11 can function as a chloride channel that is active under basal conditions and is not regulated by calcium, forskolin, or co-expression with cystic fibrosis transmembrane regulator. Single and double immunofluorescent labeling studies demonstrated the localization of vacuolar (V) H+-ATPase and Slc26a11 (KBAT) in the plasma membrane in Purkinje cells. Functional studies in HEK293 cells indicated that transfection with Slc26a11 stimulated acid transport via endogenous V H+-ATPase. We conclude that Slc26a11 (KBAT) is prominently distributed in output neurons of various subcortical and cortical structures in the central nervous system, with specific expression in Purkinje cells and that it may operate as a chloride channel regulating acid translocation by H+-ATPase across the plasma membrane and in intracellular compartments.







Similar content being viewed by others
References
Alper SL, Natale J, Gluck S, Lodish HF, Brown D (1989) Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A 86(14):5429–5433
Amlal H, Goel A, Soleimani M (1998) Activation of H+-ATPase by hypotonicity: a novel regulatory mechanism for H+ secretion in IMCD cells. Am J Physiol 275(4 Pt 2):F487–F501
Bellemer A, Hirata T, Romero MF, Koelle MR (2011) Two types of chloride transporters are required for GABA(A) receptor-mediated inhibition in C. elegans. EMBO J 30(9):1852–1863
Berend K, van Hulsteijn LH, Gans RO (2012) Chloride: the queen of electrolytes? Eur J Intern Med 23(3):203–211
Blake-Palmer KG, Karet FE (2009) Cellular physiology of the renal H+ ATPase. Curr Opin Nephrol Hypertens 18(5):433–438, Review
Brown D, Hirsch S, Gluck S (1988) Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 82(6):2114–2126
Burge JA, Hanna MG (2012) Novel insights into the pathomechanisms of skeletal muscle channelopathies. Curr Neurol Neurosci Rep 12(1):62–69, Review
Chang MH, Plata C, Zandi-Nejad K, Sindić A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF (2009) Slc26a9—anion exchanger, channel and Na+ transporter. J Membr Biol 228(3):125–140
De Zeeuw C, Hoebeek F, Bosman L et al (2011) Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci 12:327–344
Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S (2007) SLC26A9 is a Cl− channel regulated by the WNK kinases. J Physiol 584:333–345
Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED (1997) Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 17:411–422
Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg M-L, Airola K, Holmberg C, de la Chapelle A, Kere J (1996) Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhea. Nat Genet 14:316–319
Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82(2):503–568
Karlsson U, Druzin M, Johansson S (2011) Cl− concentration changes and desensitization of GABAA and glycine receptors. J Gen Physiol 138:609–626
Kasper D, Planells-Cases R, Fuhrmann JC, Olaf S, Oliver Z, Klaus R, Schmitt A, Mallorie P, Robert S, Michaela S, Uwe K, Jentsch TJ (2005) Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J 24(5):1079–1091
Kim KH, Shcheynikov N, Wang Y, Muallem S (2005) SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem 280:6463–6470
Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J (2000) Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics 70:102–112
Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J (2002) Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J Biol Chem 277:14246–14254
Lynch J (2003) Molecular structure and function of the glycine receptor chloride channel. J Physiol Rev 84:1051–1095
Marshansky V, Futai M (2008) The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20(4):415–426, Review
Martins JR, Faria D, Kongsuphol P, Reisch B, Schreiber R, Kunzelmann K (2011) Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc Natl Acad Sci U S A 108(44):18168–18172
Moriyama Y, Maeda M, Futai M (1992) The role of V-ATPase in neuronal and endocrine systems. J Exp Biol 172:171–178
Moriyama Y, Tsai HL, Futai M (1993) Energy-dependent accumulation of neuron blockers causes selective inhibition of neurotransmitter uptake by brain synaptic vesicles. Arch Biochem Biophys 305:278–281
Mulberg A, Resta L, Windner E et al (1994) Expression and localization of the cystic fibrosis transmembrane conductance regulator mRNA and its protein in rat brain. J Clin Invest 96:646–652
Murata Y, Sun-Wada GH, Yoshimizu T, Yamamoto A, Wada Y, Futai M (2002) Differential localization of the vacuolar H+ pump with G subunit isoforms (G1 and G2) in mouse neurons. J Biol Chem 277(39):36296–36303
Nelson N, Perzov N, Cohen A, Hagai K, Padler V, Nelson H (2000) The cellular biology of proton-motive force generation by V-ATPases. J Exp Biol 203(Pt 1):89–95, Review
Ohana E, Yang D, Shcheynikov N, Muallem S (2009) Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587(Pt 10):2179–2185, Review
Okada Y, Sato K, Numata T (2009) Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 587(Pt 10):2141–2149, Review
Petrovic S, Ju X, Barone S, Seidler U, Alper SL, Lohi H, Kere J, Soleimani M (2003) Identification of a basolateral Cl-/HCO3- exchanger specific to gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 284(6):G1093–G1103
Plans V, Rickheit G, Jentsch TJ (2009) Physiological roles of CLC Cl(−)/H (+) exchangers in renal proximal tubules. Pflugers Arch 458(1):23–37, Review
Poëa-Guyon S, Amar M, Fossier P, Morel N (2006) Alternative splicing controls neuronal expression of v-ATPase subunit a1 and sorting to nerve terminals. J Biol Chem 281(25):17164–17172
Pouille F, Scanziani M (2001) Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293:1159–1163
Ratte´ S, Prescott S (2011) ClC-2 channels regulate neuronal excitability, not intracellular chloride levels. J Neurosci 31(44):15838–15843
Rivera C, Voipio J, Kaila K (2005) Two developmental switches in GABAergic signaling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol 562(1):27–36
Romero MF, Chang MH, Plata C, Zandi-Nejad K, Mercado A, Broumand V, Sussman CR, Mount DB (2006) Physiology of electrogenic SLC26 paralogues. Novartis Found Symp 273:126–138
Saroussi S, Nelson N (2009) Vacuolar H(+)-ATPase-an enzyme for all seasons. Pflugers Arch 457(3):581–587, Review
Saroussi S, Nelson N (2009) The little we know on the structure and machinery of V-ATPase. J Exp Biol 12:1604–1610, Review
Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M (2006) Slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281:37962–37971
Seja P, Schonewille M, Spitzmaul G et al (2012) Raising cytosolic Cl− in cerebellar granule cells affects their excitability and vestibulo-ocular learning. EMBO I 31:1217–1230
Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE (2001) Pendrin: an apical Cl-/OH-/HCO3- exchanger in the kidney cortex. Am J Physiol Ren Physiol 280:F356–F364
Soleimani M, Xu J (2006) SLC26 chloride/base exchangers in the kidney in health and disease. Semin Nephrol 26(5):375–385
Stadler H, Tsukita S (1984) Synaptic vesicles contain an ATP-dependent proton pump and show 'knob-like' protrusions on their surface. EMBO J 3:3333–3337
Strauss O, Neussert R, Müller C, Milenkovic VM (2012) A potential cytosolic function of bestrophin-1. Adv Exp Med Biol 723:603–610, Review
Takahashi KI, Copenhagen DR (1996) Modulation of neuronal function by intracellular pH. Neurosci Res 24(2):109–116
Tornberg J, Voikar V, Savilahti H et al (2005) Behavioral phenotype of hypomorphic KCC2-deficient mice. J Eur J NeuroSci 21:1327–1337
Vincourt JB, Jullien D, Amalric F, Girard JP (2003) Molecular and functional characterization of SLC26A11, a sodium-independent sulfate transporter from high endothelial venules. FASEB J 17:890–892
Walcott BP, Kahle KT, Simard JM (2012) Novel treatment targets for cerebral edema. Neurotherapeutics 9(1):65–72, Review
Wellhauser L, D'Antonio C, Bear CE (2010) ClC transporters: discoveries and challenges in defining the mechanisms underlying function and regulation of ClC-5. Pflugers Arch 460(2):543–557, Review
Weyler R, Yurko-Mauro K, Rubenstein R et al (1999) CFTR is functionally active in GnRH-expressing GT1-7 hypothalamic neurons. Am J Physiol Cell Physiol 277:C563–C571
Wulff P, Schonewille M, Renzi M, Viltono L, Sassoè-Pognetto M, Badura A, Gao Z, Hoebeek FE, van Dorp S, Wisden W, Farrant M, De Zeeuw CI (2009) Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat Neurosci 12(8):1042–1049
Xu J, Song P, Nakamura S, Miller M, Barone S, Alper SL, Riederer B, Bonhagen J, Arend LJ, Amlal H, Seidler U, Soleimani M (2009) Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J Biol Chem 284(43):29470–29479
Xu J, Barone S, Li H, Holiday S, Zahedi K, Soleimani M (2011) Slc26a11, a chloride transporter, localizes with the vacuolar H(+)-ATPase of A-intercalated cells of the kidney. Kidney Int 80(9):926–937
Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, Ehrenfeld J, Seideler U, Soleimani M (2008) Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci U S A 105(46):17955–17960
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455(7217):1210–1215
Yao G, Feng H, Cai Y, Qi W, Kong K (2007) Characterization of vacuolar-ATPase and selective inhibition of vacuolar-H(+)-ATPase in osteoclasts. Biochem Biophys Res Commun 357(4):821–827, Review
Young A, Chu D (1990) Distribution of GABAA and GABAB receptors in mammalian brain: potential targets for drug development. J Drug Dev Res 21:161–167
Zhang Z, Nguyen KT, Barrett EF, David G (2010) Vesicular ATPase inserted into the plasma membrane of motor terminals by exocytosis alkalinizes cytosolic pH and facilitates endocytosis. Neuron 68(6):1097–1108
Acknowledgments
The authors express appreciation to M. Rutteman and E. Haasdijk from Erasmus MC for their contribution and to Dr. D. Jaarsma from Erasmus and Dr. Masato Nakafuku, Professor of Pediatrics, Division of Developmental Biology, Cincinnati Children's Hospital Research Foundation, for their helpful discussions. These studies were supported by the Dutch Organization for Medical Sciences (ZonMw; CIDZ), DFG SFB699A7 (KK), National Institute of Health R56DK62809 (MS), Life Sciences (ALW; CIDZ), Senter (Neuro-Basic; CIDZ), Merit Review Award from the Department of Veterans Administration (MS), Prinses Beatrix Fonds (CIDZ), ERCadvanced, CEREBNET and C7 programs of the European Community (CIDZ), and funds from US Renal Care (MS) and Center on Genetics of Transport at University of Cincinnati (MS).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rahmati, N., Kunzelmann, K., Xu, J. et al. Slc26a11 is prominently expressed in the brain and functions as a chloride channel: expression in Purkinje cells and stimulation of V H+-ATPase. Pflugers Arch - Eur J Physiol 465, 1583–1597 (2013). https://doi.org/10.1007/s00424-013-1300-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1300-6