Abstract
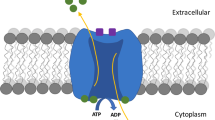
Phospholamban (PLN) is a 52 amino acid integral membrane protein of the sarcoplasmic reticulum (SR) that exists in both monomeric and pentameric forms. In its unphosphorylated state, PLN inhibits the SR Ca2+ ATPase (SERCA). This inhibition is relieved when PLN is phosphorylated as a result of β-adrenergic stimulation of the heart. Consistent with some predictions from molecular models and from functional studies of PLN incorporated into planar lipid bilayers, it has also been postulated that pentameric PLN can also form ion-selective channels. Other molecular models contradict this hypothesis, however. In the work reported here, we used the Ca2+-sensitive fluorescent dye Fura-2, to examine the passive Ca2+ permeability of the SR membrane in vesicles derived from cardiac ventricle. We have found that phosphorylation of PLN by protein kinase A (PKA) leads to an increase in the rate of Ca2+ leak from Ca2+-loaded SR vesicles. This enhanced rate of Ca2+ leak from the SR is also observed when SR vesicles are incubated with a PLN specific antibody (A1) that mimics phosphorylation of PLN. The ryanodine receptor blocker ruthenium red does not affect the increased rate of Ca2+ leak from the SR after PLN phosphorylation with PKA or after exposure to A1 antibody, arguing against a possible role of ryanodine receptors in mediating the enhanced leak. Our results are consistent with the hypothesis that phosphorylated PLN forms or regulates a Ca2+ leak pathway in cardiac SR membranes in situ.




Similar content being viewed by others
References
Adams PD, Arkin IT, Engelman DM, Brunger AT (1995) Computational searching and mutagenesis suggest a structure for the pentameric transmembrane domain of phospholamban. Nat Struct Biol 2:154–162
Arkin IT, Adams PD, MacKenzie KR, Lemmon MA, Brunger AT, Engelman DM (1994) Structural organization of the pentameric transmembrane alpha-helices of phospholamban, a cardiac ion channel. EMBO J 13:4757–4764
Arkin IT, Rothman M, Ludlam CF, Aimoto S, Engelman DM, Rothschild KJ, Smith SO (1995) Structural model of the phospholamban ion channel complex in phospholipid membranes. J Mol Biol 248:824–834
Bazzazi H, Kargacin ME, Kargacin GJ (2003) Ca2+ regulation in the near-membrane microenvironment in smooth muscle cells. Biophys J 85:1754–1765
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 415:198–205
Camello C, Lomax R, Petersen OH, Tepikin AV (2002) Calcium leak from intracellular stores—the enigma of calcium signaling. Cell Calcium 32:355–361
Carter S, Pitt SJ, Colyer J, Sitsapesan R (2011) Ca²+-dependent phosphorylation of RyR2 can uncouple channel gating from direct cytosolic Ca²+ regulation. Membr Biol 240:21–33
Cerra MC, Imbrogno S (2011) Phospholamban and cardiac function: a comparative perspective in vertebrates. Act Physiol Nov 30. doi:10.1111/j.1748-1716.2011.02389 Epub
Chamberlain BK, Volpe P, Fleischer S (1984) Inhibition of calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. J Biol Chem 259:7547–7553
Decrouy A, Juteau M, Proteau S, Teijiera J, Rousseau E (1996) Biochemical regulation of sarcoplasmic reticulum Cl− channel from human atrial myocytes: involvement of phospholamban. J Mol Cell Cardiol 28:767–780
Fujii J, Kadoma M, Tada M, Toda H, Sakiyama F (1986) Characterization of structural unit of phospholamban by amino acid sequencing and electrophoretic analysis. Biochem Biophys Res Commun 138:1044–1050
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Hou Z, Kelly EM, Robia SL (2008) Phosphomimetic mutations increase phospholamban oligomerization and alter the structure of its regulatory complex. J Biol Chem 283:28996–29003
Inesi G, Prasad AM, Pilankatta R (2008) The Ca2+ ATPase of cardiac sarcoplasmic reticulum: physiological role and relevance to diseases. Biochem Biophys Res Commun 369:182–187
Kargacin GJ (1994) Calcium signaling in restricted diffusion spaces. Biophys J 67:262–272
Kargacin GJ (2003) Responses of Ca2+-binding proteins to localized, transient changes in intracellular [Ca2+]. J Theor Biol 221:245–258
Kargacin ME, Ali Z, Kargacin GJ (1998) Anti-phospholamban and PKA alter the Ca2+ sensitivity and V max of Ca2+ uptake by the cardiac SR. Biochem J 331:245–249
Kargacin ME, Emmett TL, Kargacin GJ (2011) Epigallocatechin-3-gallate has dual, independent effects on the cardiac sarcoplasmic reticulum/endoplasmic reticulum Ca2+ ATPase. J Muscle Res Cell Motil 32:89–98
Kargacin GJ, Fay FS (1991) Ca2+ movement in smooth muscle cells studied with one and two dimensional diffusion models. Biophys J 60:1088–1100
Kargacin ME, Kargacin GJ (1994) Methods for determining cardiac sarcoplasmic reticulum Ca2+ pumping kinetics from fura 2 measurements. Am J Physiol 267:C1145–C1151
Kargacin ME, Scheid CR, Honeyman TW (1988) Continuous monitoring of Ca2+ uptake in membrane vesicles with fura-2. Am J Physiol 255:C694–C698
Kovacs RJ, Nelson MT, Simmerman HK, Jones LR (1988) Phospholamban forms Ca2+-selective channels in lipid bilayers. J Biol Chem 263:18364–18368
Laver DR, Owen VJ, Junankar PR, Taske NL, Dulhunty AF, Lamb GD (1997) Reduced inhibitory effect of Mg2+ on ryanodine receptor-Ca2+ release channels in malignant hyperthermia. Biophys J 73:1913–1924
Lucchesi PA, Cooney RA, Mangsen-Baker C, Honeyman TW, Scheid CR (1988) Assessment of transport capacity of plasmalemmal Ca2+ pump in smooth muscle. Am J Physiol 255:C226–C236
Lytton J, Westlin M, Hanley MR (1991) Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266:17067–17071
Ma J (1993) Block by ruthenium red of the ryanodine-activated calcium release channel of skeletal muscle. J Gen Physiol 102:1031–1056
Maffeo C, Aksimentiev A (2009) Structure, dynamics, and ion conductance of the phospholamban pentamer. Biophys J 96:4853–4865
Makinose M, Hasselbach W (1971) ATP synthesis by the reverse of the sarcoplasmic calcium pump. FEBS Lett 12:271–272
Meissner G, Fleischer S (1974) Reconstitution of functional sarcoplasmic reticulum membrane vesicles. Methods Enzym 32:475–481
Michailova A, McCulloch A (2001) Model study of ATP and ADP buffering, transport of Ca2+ and Mg2+, and regulation of ion pumps in ventricular myocyte. Biophys J 81:614–629
Moncoq K, Trieber CA, Young HS (2007) The molecular basis for cyclopiazonic acid inhibition of the sarcoplasmic reticulum calcium pump. J Biol Chem 282:9748–9757
Morris GL, Cheng HC, Colyer J, Wang JH (1991) Phospholamban regulation of cardiac sarcoplasmic reticulum (Ca2+-Mg2+)-ATPase. Mechanism of regulation and site of monoclonal antibody interaction. J Biol Chem 266:11270–11275
Oxenoid K, Chou JJ (2005) The structure of phospholamban pentamer reveals a channel-like architecture in membranes. Proc Natl Acad Sci USA 102:10870–10875
Ozawa T (2010) Modulation of ryanodine receptor Ca2+ channels (review). Mol Med Report 3:199–204
Sammels E, Parys JB, Missiaen L, De Smedt H, Bultynck G (2010) Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium 47:297–314
Shannon TR, Chu G, Kranias EG, Bers DM (2001) Phospholamban decreases the energetic efficiency of the sarcoplasmic reticulum Ca pump. J Biol Chem 276:7195–7201
Shannon TR, Ginsburg KS, Bers DM (2000) Reverse mode of the sarcoplasmic reticulum calcium pump and load-dependent cytosolic calcium decline in voltage-clamped cardiac ventricular myocytes. Biophys J 78:322–333
Simmerman HK, Kobayashi YM, Autry JM, Jones LR (1996) A leucine zipper stabilizes the pentameric membrane domain of phospholamban and forms a coiled-coil pore structure. J Biol Chem 271:5941–5946
Sipido KR, Wier WG (1991) Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation–contraction coupling. J Physiol 435:605–630
Suzuki T, Wang JH (1986) Stimulation of bovine cardiac sarcoplasmic reticulum Ca2+ pump and blocking of phospholamban phosphorylation and dephosphorylation by a phospholamban monoclonal antibody. J Biol Chem 261:7018–7023
Takahashi M, Kondou Y, Toyoshima C (2007) Interdomain communication in calcium pump as revealed in the crystal structures with transmembrane inhibitors. Proc Natl Acad Sci USA 104:5800–5805
Takenaka H, Adler PN, Katz AM (1982) Calcium fluxes across the membrane of sarcoplasmic reticulum vesicles. J Biol Chem 257:12649–12656
ter Keurs HE, Wakayama Y, Sugai Y, Price G, Kagaya Y, Boyden PA, Miura M, Stuyvers BD (2006) Role of sarcomere mechanics and Ca2+ overload in Ca2+ waves and arrhythmias in rat cardiac muscle. Ann N Y Acad Sci 1080:248–267
Thomas DD, Reddy LG, Karim CB, Li M, Cornea R, Autry JM, Jones LR, Stamm J (2000) Direct spectroscopic detection of molecular dynamics and interactions of the calcium pump and phospholamban. Ann N Y Acad Sci 853:186–194
Toyoshima C, Nomura H (2002) Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418:605–611
Vafiadaki E, Papalouka V, Arvanitis DA, Kranias EG, Sanoudou D (2009) The role of SERCA2a/PLN complex, Ca2+ homeostasis, and anti-apoptotic proteins in determining cell fate. Pflugers Arch 457:687–700
Vassalle M, Lin CI (2004) Calcium overload and cardiac function. J Biomed Sci 11:542–565
Verardi R, Shi L, Traaseth NJ, Walsh N, Veglia G (2011) Structural topology of phospholamban pentamer in lipid bilayers by a hybrid solution and solid-state NMR method. Proc Natl Acad Sci USA 108:9101–9106
Wegener AD, Jones LR (1984) Phosphorylation-induced mobility shift in phospholamban in sodium dodecyl sulfate-polyacrylamide gels. Evidence for a protein structure consisting of multiple identical phosphorylatable subunits. J Biol Chem 259:1834–1841
Worsfold M, Peter JB (1970) Kinetics of calcium transport by fragmented sarcoplasmic reticulum. J Biol Chem 245:5545–5552
Xu L, Mann G, Meissner G (1996) Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ Res 79:1100–1109
Zima AV, Bovo E, Bers DM, Blatter LA (2010) Ca²+ spark-dependent and-independent sarcoplasmic reticulum Ca²+ leak in normal and failing rabbit ventricular myocytes. J Physiol 588:4743–4757
Acknowledgements
This work was supported by grants from the National Science and Engineering Council of Canada and the Heart and Stroke Foundation of Alberta/Northwest Territories.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gary J. Kargacin and Margaret E. Kargacin contributed equally to the work reported in this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Determination of Ca2+ release rates from Ca2+ loaded cardiac SR vesicles. A Fura-2 340/380 fluorescence ratio values after subtraction of background light for an experiment examining Ca2+ uptake and release from cardiac SR vesicles. Vesicles were actively loaded with Ca2+ by addition of ATP (left arrow) to activate SERCA-dependant Ca2+ transport (note: the 340/380 ratio decreases during uptake because Fura-2 was present in the extravesicular buffer). Passive Ca2+ release from the vesicles is initiated after SERCA activity was stopped by addition of thapsigargin (right arrow). Green curve is a double exponential fit to the data from the release portion of the experiment. B Portion of the release fit from the green curve in A for the first 50 s of release. C Portion of the release fit from the first 5 s of release (filled circles) showing that the initial Ca2+ release rate in the experiments can be determined from a linear fit (line) to the initial portion of a release curve. D Experiment to determine if there was a detectable delay in the effect of thapsigargin on SERCA activity in our experiments (see text for details). Thapsigargin was added (arrow) at ∼52 s. The 340/380 ratio values < 5 are calculated from the dark current signals recorded when the shutter was closed to add thapsigargin. The result indicates that thapsigargin had mixed completely and its maximum effect on SERCA activity was reached during the time taken for its addition to the cuvette. (PPTX 170 kb)
Supplemental Fig. 2
Phosphorylation of PLN with cPKA. A Western blot of cardiac SR vesicle proteins incubated with A1 antibody. B Western blot of cardiac SR vesicle proteins incubated with phospho-serine16 specific PLN antibody (PS-16). In A and B: lane 1, incubation with cPKA + ATPγS; lane 2, incubation with boiled cPKA + ATPγS; lane 3, incubation with ATPγS alone; lane 4, incubation with cPKA + ATP. Approximate molecular weights are shown to the left of the blots. Results indicate that both monomeric (∼6 kDa) and pentameric (∼26 kDa) forms of PLN are present in the vesicle samples and that both phosphorylated and thiophosphorylated PLN can be detected with PS-16. Note the slight shift in mobility of PLN in lanes 1 and 4 in A indicative of phosphorylation which is confirmed in B by PS-16. For the experiments shown, samples from a cardiac SR vesicle preparation were incubated with the phosphorylation components as described in Methods for the uptake experiments. After incubation, proteins in the samples were precipitated by adding an equal volume of 25% trichloroacetic acid to the samples. They were then incubated on ice for 30 min, centrifuged and resuspended into 90 μl of 20 mM Tris (pH 8.5) and an equal volume of 2 x Laemmli sample buffer prior to electrophoresis. Samples (10 μg protein) were loaded in each lane and proteins were separated on 15% polyacrylamide gels and transferred to PVDF membranes for detection. (PPTX 322 kb)
Rights and permissions
About this article
Cite this article
Aschar-Sobbi, R., Emmett, T.L., Kargacin, G.J. et al. Phospholamban phosphorylation increases the passive calcium leak from cardiac sarcoplasmic reticulum. Pflugers Arch - Eur J Physiol 464, 295–305 (2012). https://doi.org/10.1007/s00424-012-1124-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1124-9