Abstract
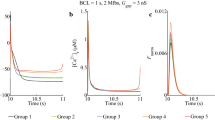
Membrane potential changes of atrial fibroblasts in response to mechanical stress have been considered to modulate the rhythmic electrical activity of healthy hearts. Our recent findings suggest that cardiac arrhythmia after infarction is related to enhanced susceptibility of the fibroblasts to physical stretch. In this study, we analysed the effect of hypoxia/reoxygenation, which are major components of tissue ischemia/reperfusion, on the membrane potential of atrial fibroblasts. Intracellular microelectrode recordings were performed together with isometric force measurements on isometrically contracting right atrial tissue preparations from adult rats. Lowering the oxygen tension in the perfusate from 80 kPa to 3.5 kPa reduced active force development and decreased the resting membrane potential of the cardiac fibroblasts from −23±5 mV to −5±2 mV (n=35). Application of gadolinium (40 μM) to inhibit non-selective cation channels prevented hypoxia-induced membrane depolarization of the fibroblasts. Reoxygenation of the myocardial tissue resulted in a transient increase of the resting membrane potential to maximally −60±8 mV. These findings indicate that transmembrane currents in atrial fibroblasts are sensitive to changes in tissue oxygenation. In conclusion, altered electro-mechanical function of the ischemic heart may possibly involve changes of the membrane potential of the cardiac fibroblasts.




Similar content being viewed by others
References
Camelliti P, Kohl P, Green C (2002) Myocyte/non-myocyte interactions in the heart: clues to an alternative mechanosensor for cardiac MEF. In: Proceedings of the Physiological Society, University of Leeds, 10–12 September, 21P
Duncan CJ (1978) Role of intracellular calcium in promoting muscle damage: a strategy for controlling the dystrophic condition. Experimentia 34:1531–1535
Dzau VJ (1993) Local contractile and growth modulators in the myocardium. Clin Cardiol 16 [Suppl 2]:II5–II9
Eghbali M (1992) Cardiac fibroblasts: function, regulation of gene expression and phenotypic modulation. Basic Res Cardiol 87:183–189
French AS, Stockbridge LL (1988) Potassium channels in human and avian fibroblasts. Proc R Soc Lond B 232:395–412
Jennings RB, Reimer KA, Steenbergen C (1985) Myocardial ischemia and reperfusion: role of calcium. In: Parratt JR (ed) Control and manipulation of calcium movement. Raven, New York, pp 273–302
Kamkin A, Kiseleva I, Isenberg G (2003) Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc Res (in press)
Kamkin A, Kiseleva I, Wagner KD, Lammerich A, Bohm J, Persson PB, Günther J (1999) Mechanically induced potentials in fibroblasts from human right atrium. Exp Physiol 84:347–356
Kamkin A, Kiseleva I, Wagner KD, Pylaev A, Leiterer KP, Theres H, Scholz H, Günther J, Isenberg G (2002) A possible role for atrial fibroblasts in postinfarction bradycardia. Am J Physiol 282:H842–H849
Kiseleva I, Kamkin A, Kohl P, Lab MJ (1996) Calcium and mechanically induced potentials in fibroblasts of rat atrium. Cardiovasc Res 32:98–111
Kiseleva I, Kamkin A, Pylaev A, Kondratjev D, Leiterer KP, Theres H, Wagner KD, Persson PB, Günther J (1998) Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. J Mol Cell Cardiol 30:1083–1093
Kohl P (1995) Mechano-electric feedback impact on heart rhythm. Futura 4:240–252
Kohl P, Kamkin A, Kiseleva I, Noble D (1994) Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol 79:943–956
Kohl P, Kamkin A, Kiseleva I, Streubel T (1992) Mechanosensitive cells in the atrium of frog heart. Exp Physiol 77:213–216
Kohl P, Noble D (1996) Mechanosensitive connective tissue: potential influence on heart rhythm. Cardiovasc Res 32:62–68
Lab MJ (1996) Mechanoelectric feedback (transduction) in heart: concepts and implication. Cardiovasc Res 32:3–14
Lammerich A, Bohm J, Schimke I, Wagner KD, Storch E, Günther J (1996) Effects of hypoxia, simulated ischemia and reoxygenation on the contractile function of human atrial trabeculae. Mol Cell Biochem 160/161:143–151
Naccarella F, Lepera G, Rolli A (2000) Arrhythmic risk stratification of post-myocardial infarction patients. Curr Opin Cardiol 15:1–6
Nayler WG, Poole-Wison PA, Williams A (1979) Hypoxia and calcium. J Mol Cell Cardiol 11:683–706
Nazir SA, Lab MJ (1996) Mechanoelectric feedback in the atrium of the isolated guinea-pig heart. Cardiovasc Res 32:112–119
Okada Y, Oiki T, Ohno-Shosaku S, Ueda S, Yada T (1986) Intracellular Ca2+ and calmodulin regulate K+ conductance in cultured fibroblasts. Biomed Res 7S:73–78
Rook MB, van Ginneken ACG, de Jonge B, El Aoumari A, Gros D, Jongsma HJ (1992) Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol 263:C959–C977
Underwood RD, Sra J, Akhtar M (1997) Evaluation and treatment strategies in patients at high risk of sudden death post myocardial infarction. Clin Cardiol 20:753–758
Wagner KD, Theres H, Born A, Strube S, Wunderlich N, Pfitzer G, Baumann G, Günther J (1997) Contractile function of papillary muscle from rats with different infarct size after β-adrenergic blockade and ACE-inhibition. J Mol Cell Cardiol 29:2941–2951
Ward H, White E (1994) Reduction in the contraction and intracellular calcium transient of single rat ventricular myocytes by gadolinium and the attenuation of these effects by extracellular NaH2PO4. Exp Physiol 79:107–110
Acknowledgements
This study was financially supported by grants from the Humboldt-University of Berlin and the Alexander von Humboldt-Stiftung. A.K. was a fellow of the Alexander von Humboldt-Stiftung, and I.K. received a travel grant from the Humboldt-University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamkin, A., Kiseleva, I., Wagner, KD. et al. Mechanically induced potentials in atrial fibroblasts from rat hearts are sensitive to hypoxia/reoxygenation. Pflugers Arch - Eur J Physiol 446, 169–174 (2003). https://doi.org/10.1007/s00424-003-1032-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-003-1032-0