Abstract
Background
The number of lesions and the size of the largest (CRLMmax) have been widely investigated as prognostic factors in patients with colorectal liver metastases (CRLM). The aim of the present study was to assess whether, in patients undergoing curative liver resection, the presence of infracentimetric lesions could affect recurrence-free survival (RFS) and overall survival (OS).
Methods
Patients who underwent a liver resection for CRLM between 2001 and 2019 were included. The size of CRLM was measured on the surgical specimen. The best cut-off of the smallest lesion (CRLMmin) associated with RFS was determined through the time-dependent ROC analysis. A multivariate Cox regression analysis was carried out.
Results
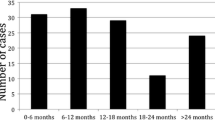
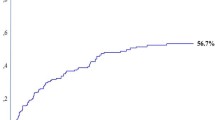
Overall, 227 patients were included. Median follow-up time was 50 months [IQR 26–84]. Recurrence occurred for 151 (66.5%) patients (liver recurrence in 67.5%, while exclusive extra-hepatic recurrence in 32.5%). The best cut-off for CRLMmin associated with RFS was 9 mm, with 12- and 24-month td-AUC 0.56 and 0.52 respectively. CRLMmin ≤ 9 mm was found to be an independent prognostic factor that impairs RFS at multivariate analysis (HR 1.534 (1.02–2.32), p = 0.042). In particular, CRLMmin ≤ 9 mm was correlated with impaired hepatic RFS (HR 1.860 (1.15–3.01), p = 0.011), but not extra-hepatic RFS.
Conclusions
Infracentimetric metastases (≤ 9 mm) are an independent prognostic factor that impairs hepatic RFS. This result suggests the potential benefit of neoadjuvant chemotherapy (CT) also in selected patients with initially resectable lesions, in case of CRLM ≤ 9 mm on preoperative imaging.






Similar content being viewed by others
Data Availability
Data available on request from the authors.
Abbreviations
- CRLM:
-
Colorectal liver metastases
- CRLMmax:
-
Largest colorectal liver metastasis
- CRLMmin:
-
Smallest colorectal liver metastasis
- RFS:
-
Recurrence-free survival
- OS:
-
Overall survival
- ROC:
-
Receiving operating curve
- FLR:
-
Future liver remnant
- CT-scan:
-
Computed tomography
- MR:
-
Magnetic resonance
- US:
-
Ultrasound
- CT:
-
Chemotherapy
References
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244:254–259
Pawlik TM, Schulick RD, Choti MA (2008) Expanding criteria for resectability of colorectal liver metastases. Oncologist 13:51–64
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, Levi F, Bismuth H (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term servival. Ann Surg 240:644–657
Viganò L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, Ijzermans JN, Mirza DF, Elias D, Adam R (2014) Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol 21:1276–1286
Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D (1996) Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie Cancer 77:1254–1262
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318
Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, Geller DA, Gayowski TJ, Fung JJ, Starzl TE (1999) Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 189:291–299
Nagashima I, Takada T, Matsuda K, Adachi M, Nagawa H, Muto T, Okinaga K (2004) A new scoring system to classify patients with colorectal livermetastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg 11:79–83
Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG (2008) Evaluation oflongterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247:125–135
Gomez D, Cameron IC (2010) Prognostic scores for colorectal liver metastasis: clinically important or an academic exercise? HPB (Oxford) 12:227–238
Ko Y, Kim J, Park JK, Kim H, Cho JY, Kang SB, Ahn S, Lee KJ, Lee KH (2017) Limited detection of small (≤ 10 mm) colorectal liver metastasis at preoperative CT in patients undergoing liver resection. PLoS One. 12(12):e0189797
Adam R, Wicherts DA, de Haas RJ, Aloia T, Lévi F, Paule B, Guettier C, Kunstlinger F, Delvart V, Azoulay D, Castaing D (2008) Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol 26:1635–1641
Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, Vauthey JN, Påhlman L (2015) Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev 41:729–741
Okumura S, Tabchouri N, Leung U, Tinguely P, Louvet C, Beaussier M, Gayet B, Fuks D (2019) Laparoscopic parenchymal-sparing hepatectomy for multiple colorectal liver metastases improves outcomes and salvageability: a propensity score-matched analysis. Ann Surg Oncol 26:4576–4586
Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, Makuuchi M, Strong RW (2000) The Brisbane 2000 terminology of liver anatomy and resections. HPB 2:333–339
Gayet B, Cavaliere D, Vibert E, Perniceni T, Levard H, Denet C, Christidis C, Blain A, Mal F (2007) Totally laparoscopic right hepatectomy. Am J Surg 194:685–689
Gumbs AA, Gayet B (2007) Totally laparoscopic left hepatectomy. Surg Endosc 21:1221
Gumbs AA, Bar-Zakai B, Gayet B (2008) Totally laparoscopic extended left hepatectomy. J Gastrointest Surg 12:1152
Gumbs AA, Gayet B (2008) Totally laparoscopic extended right hepatectomy. Surg Endosc 22:2076–2077
Ishizawa T, Gumbs AA, Kokudo N, Gayet B (2012) Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 256:959–964
Altendorf-Hofmann A, Scheele J (2003) A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am 12(1):165–192
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G (2012) Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 4:283–301
Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, Kumamoto T, Iacono C, Andreatos N, Guglielmi A, Endo I, Pawlik TM (2018) The Tumor Burden Score: a new “metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg 267:132–141
Acciuffi S, Meyer F, Bauschke A, Settmacher U, Lippert H, Croner R, Altendorf-Hofmann A (2018) Analysis of prognostic factors after resection of solitary liver metastasis in colorectal cancer: a 22-year bicentre study. J Cancer Res Clin Oncol 144:593–599
Tabchouri N, Gayet B, Okumura S, Donatelli G, Beaussier M, Bennamoun M, Louvet C, Fuks D (2018) Recurrence patterns after laparoscopic resection of colorectal liver metastases. Surg Endosc 32:4788–4797
Taylor M, Forster J, Langer B, Taylor BR, Greig PD, Mahut C (1997) A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg 173:467–471
Niekel MC, Bipat S, Stoker J (2010) Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257:674–684
Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aubé C, Chatellier G (2016) A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur Radiol 26:4595–4615
Tanaka K, Takakura H, Takeda K, Matsuo K, Nagano Y, Endo I (2009) Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg 250:935–942
Gaujoux S, Goéré D, Dumont F, Souadka A, Dromain C, Ducreux M, Elias D (2011) Complete radiological response of colorectal liver metastases after chemotherapy: what can we expect? Dig Surg 28:114–120
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. In particular, material preparation, data collection and analysis were performed by Fabio Frosio and Baptiste Cervantes. The first draft of the manuscript was written by Fabio Frosio and all authors commented on previous versions of the manuscript. Christophe Louvet, Brice Gayet and David Fuks revised it critically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frosio, F., Cervantes, B., Nassar, A. et al. Prognostic role of infracentimetric colorectal liver metastases. Langenbecks Arch Surg 407, 1971–1980 (2022). https://doi.org/10.1007/s00423-022-02499-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02499-4