Abstract
Purpose
Accumulation of ammonia causes central and peripheral fatigue. This study aimed to investigate the synergistic effect of tea catechins and low-dose ornithine in activating the urea cycle to reduce blood ammonia levels during exercise.
Methods
We used hepatocyte-like cells derived from human-induced pluripotent stem (iPS) cells to assess the effect of tea catechins combined with ornithine on urea cycle activity. The urea production and expression of key genes involved in the metabolism of urea were investigated. We then examined the synergistic improvement in ammonia metabolism by tea catechins in combination with ornithine in a human pilot study.
Results
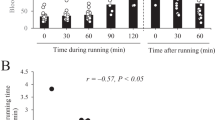
Tea catechins combined with ornithine increased urea cycle activity in hepatocyte-like cells derived from human iPS cells. Intake of 538.6 mg of tea catechins with 1592 mg of ornithine for 2 consecutive days during exercise loading suppressed the exercise-induced increase in the blood ammonia concentration as well as stabilized blood glucose levels.
Conclusion
Controlling the levels of ammonia, a toxic waste produced in the body, is important in a variety of situations, including exercise. The present study suggests that a heterogeneous combination of polyphenols and amino acids efficiently suppresses elevated ammonia during exercise in humans by a mechanism that includes urea cycle activation.
Trial registration
This study was registered in the University Hospital Medical Information Network Clinical Trial Registry (No. UMIN000035484, dated January 8, 2019).



Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARG:
-
Arginase
- ASL:
-
Argininosuccinate lyase
- ASS:
-
Argininosuccinate synthase
- AUC:
-
Area under the curve (total)
- BMI:
-
Body mass index
- cDNA:
-
Copy deoxyribonucleic acid
- CPS:
-
Carbamoyl-phosphate synthase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GOT:
-
Glutamic-oxaloacetic transaminase
- HRmax:
-
Maximum heart rate
- iPS:
-
Induced pluripotent stem cell
- mRNA:
-
Messenger ribonucleic acid
- OTC:
-
Ornithine carbamoyltransferase
- PCR:
-
Polymerase chain reaction
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- RNA:
-
Ribonucleic acid
- SD:
-
Standard deviation
- SE:
-
Standard error
- TCA:
-
Tricarboxylic acid
References
Banister EW, Cameron BJ (1990) Exercise-induced hyperammonemia: peripheral and central effects. Int J Sports Med 11:S129–S142. https://doi.org/10.1055/s-2007-1024864
Banister EW, Allen ME, Mekjavic IB, Singh AK, Legge B, Mutch BJ (1983) The time course of ammonia and lactate accumulation in blood during bicycle exercise. Eur J Appl Physiol Occup Physiol 51:195–202. https://doi.org/10.1007/BF00455182
Chen WW, Qin GY, Zhang T (2012) Feng WY (2012) In vitro drug metabolism of green tea catechins in human, monkey, dog, rat and mouse hepatocytes. Drug Metab Lett 6:73–93. https://doi.org/10.2174/187231212804096709
Chen S, Minegishi Y, Hasumura T (2020) Involvement of ammonia metabolism in the improvement of endurance performance by tea catechins in mice. Sci Rep 10:1–3. https://doi.org/10.1038/s41598-020-63139-9
Choi YS, Lee DY, Kim IY, Kang S, Ahn K, Kim HJ, Jeong YH, Chun GT, Park JK, Kim IH (2000) Ammonia removal using hepatoma cells in mammalian cell cultures. Biotechnol Prog 16:760–768. https://doi.org/10.1021/bp000099d
Davuluri G, Allawy A, Thapaliya S, Rennison JH, Singh D, Kumar A, Sandlers Y, Van Wagoner DR, Flask CA, Hoppel C, Kasumov T, Dasarathy S (2016) Hyperammonaemia-induced skeletal muscle mitochondrial dysfunctionresults in cataplerosis and oxidative stress. J. Physiol. 594:7341–7360. https://doi.org/10.1113/JP272796
Demura S, Morishita K, Yamada T, Yamaji S, Komatsu M (2011) Effect of L-ornithine hydrochloride ingestion on intermittent maximal anaerobic cycle ergometer performance and fatigue recovery after exercise. Eur J Appl Physiol 111:2837–2843. https://doi.org/10.1007/s00421-011-1896-1
Enosawa S, Suzuki S, Kakefuda T, Amemiya H (1996) Examination of 7-ethoxycoumarin demethylation and ammonia removal activities in 31 hepatocyte cell lines. Cell Transplant 5:S39–S40. https://doi.org/10.1016/0963-6897(96)00036-x
Hattori M, Kusumoto IT, Namba T, Ishigami T, Hara Y (1990) Effect of tea polyphenols on glucan synthesis by glucosyltransferase from Streptococcus mutans. Chem Pharm Bull 38:717–720. https://doi.org/10.1248/cpb.38.717
Hellsten Y, Richter EA, Kiens B, Bangsbo J (1999) AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J Physiol 520:909–920. https://doi.org/10.1111/j.1469-7793.1999.00909.x
Hibi M, Takase H, Iwasaki M, Osaki N, Katsuragi Y (2018) Efficacy of tea catechin-rich beverages to reduce abdominal adiposity and metabolic syndrome risks in obese and overweight subjects: a pooled analysis of 6 human trials. Nutr Res 55:1–10. https://doi.org/10.1016/j.nutres.2018.03.012
Jalan R, Wright G, Davies NA, Hodges SJ (2007) l-Ornithine phenylacetate (OP): A novel treatment for hyperammonemia and hepatic encephalopathy. Med Hypotheses 69:1064–1069. https://doi.org/10.1016/j.mehy.2006.12.061
Jiang X, Chang H, Zhou Y (2015) Expression, purification and preliminary crystallographic studies of human glutamate oxaloacetate transaminase 1 (GOT1). Protein Expr Purif 113:102–106. https://doi.org/10.1016/j.pep.2015.05.010
Khan N, Mukhtar H (2007) Tea polyphenols for health promotion. Life Sci 81:519–533. https://doi.org/10.1016/j.lfs.2007.06.011
Knepper MA, Packer R, Good DW (1989) Ammonium transport in the kidney. Physiol Rev 69:179–249. https://doi.org/10.1152/physrev.1989.69.1.179
Lowenstein JM (1972) Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev 52:382–414. https://doi.org/10.1152/physrev.1972.52.2.382
Lu H, Meng X, Li C, Sang S, Pattern C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS (2003) Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos 31:452–461. https://doi.org/10.1124/dmd.31.4.452
Lu J, Einhorn S, Venkatarangan L, Miller M, Mann D (2015) Morphological and functional characterization and assessment of iPSC-derived hepatocytes for in vitro toxicity testing. Toxicol Sci 147:39–54. https://doi.org/10.1093/toxsci/kfv117
Matsui Y, Kinoshita K, Osaki N, Wakisaka T, Hibi M, Katsuragi Y, Yamaguchi TF (2018) Effects of tea catechin-rich beverage on abdominal fat area and body weight in obese Japanese individuals-A randomized, double-blind, placebo-controlled, parallel-group study. Jpn Pharmacol Ther 46:1383–1395
Meijer AJ, Gimpel JA, Deleeuw G, Tischler ME, Tager JM, Williamson JR (1978) Interrelationships between gluconeogenesis and ureogenesis in isolated hepatocytes. J Biol Chem 253:2308–2320
Morita M, Iizuka M, Morishita K, Ochiai M, Yokoyama T, Watanabe F, Kowatari Y (2013) An open-label safety trial of kinetics and metabolic effects of orally-administered L-ornithine hydrochloride in healthy volunteers - A study of its effects, particularlyon plasma L-ornithine levels and retinal function. Jpn Pharmacol Ther 41:779–787. https://doi.org/10.1038/ejcn.2010.149
Murase T, Haramizu S, Shimotoyodome A, Nagasawa A, Tokimitsu I (2005) Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am J Physiol Regul Integr Comp Physiol 288:R708–R715. https://doi.org/10.1152/ajpregu.00693.2004
Murase T, Haramizu S, Shimotoyodome A, Tokimitsu I, Hase T (2006) Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am J Physiol Regul Integr Comp Physiol 290:R1550–R1556. https://doi.org/10.1152/ajpregu.00752.2005
Ota N, Soga S, Shimotoyodome A (2016) Daily consumption of tea catechins improves aerobic capacity in healthy male adults: A randomized double-blind, placebo-controlled, crossover trial. Biosci Biotechnol Biochem 80:2412–2417. https://doi.org/10.1080/09168451.2016.1224638
Qi L, Martin-Sandoval MS, Merchant S, Gu W, Eckhardt M, Mathews TP, Zhao Z, Agathocleous M, Morrison SJ (2021) Aspartate availability limits hematopoietic stem cell function during hematopoietic regeneration. Cell Stem Cell 28:1982–1999. https://doi.org/10.1016/j.stem.2021.07.011
Rashidi B, Malekzadeh M, Goodarzi M (2017) Green tea and its anti-angiogenesis effects. Biomed Pharmacother 89:949–956. https://doi.org/10.1016/j.biopha.2017.01.161
Rose CF (2012) Ammonia-lowering strategies for the treatment of hepatic encephalopathy. Clin Pharmacol Ther 92:321–331. https://doi.org/10.1038/clpt.2012.112
Saijo R, Takeda Y (1999) HPLC analysis of catechins in various kinds of green teas produced in Japan and abroad. Nippon Shokuhin Kagaku Kogaku Kaishi 46:138–147. https://doi.org/10.3136/nskkk.46.138
Saltin B, Larsen H, Terrados N, Bangsbo J, Bak T, Kim CK, Svedenhag J, Rolf CJ (1995) Aerobic exercise capacity at sea level and at altitude in Kenyan boys, junior and senior runners compared with Scandinavian runners. Scand J Med Sci Sports 5:209–221. https://doi.org/10.1111/j.1600-0838.1995.tb00037.x
Sirenko O, Hesley J, Rusyn I, Cromwell EF (2014) High-content assays for hepatotoxicity using induced pluripotent stem cell-derived cells. Assay Drug Dev Technol 12:43–54. https://doi.org/10.1089/adt.2013.520
Sugino T, Shirai T, Kajimoto Y, Kajimoto O (2008) L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism. Nutr Res 28:738–743. https://doi.org/10.1016/j.nutres.2008.08.008
Suzuki Y, Miyoshi N, Isemura M (2012) Health-promoting effects of green tea. Proc Jpn Acad Ser B Phys Biol Sci 88:88–101. https://doi.org/10.2183/pjab.88.88
Suzuki T, Pervin M, Goto S, Isemura M, Nakamura Y (2016) Beneficial effects of tea and the green tea catechin epigallocatechin-3-gallate on obesity. Molecules 21:1305. https://doi.org/10.3390/molecules21101305
Takeda K, Takemasa T (2015) Expression of ammonia transporters Rhbg and Rhcg in mouse skeletal muscle andthe effect of 6-week training on these proteins. Physiol Rep 3:e12596. https://doi.org/10.14814/phy2.12596
Umeda M, Tominaga T, Kozuma K, Kitazawa H, Furushima D, Hibi M, Yamada H (2021) Preventive effects of tea and tea catechins against influenza and acute upper respiratory tract infections: a systematic review and meta-analysis. Eur J Nutr 60:4189–4202. https://doi.org/10.1007/s00394-021-02681-2
Vaidyanathan JB, Walle T (2002) Glucuronidation and sulfation of the tea flavonoid (-)-epicatechin by the human and rat enzymes. Drug Metab Dispos 30:897–903. https://doi.org/10.1124/dmd.30.8.897
Wang Q, Guan K, Lv Y, Zhang Y, Yu Z, Kan Q (2022) Disturbance of hepatocyte growth and metabolism in a hyperammonemia microenvironment. Arch Biochem Biophys 716:109109. https://doi.org/10.1016/j.abb.2021.109109
Wilkinson DJ, Smeeton NJ, Watt PW (2010) Ammonia metabolism, the brain and fatigue; revisiting the link. Prog Neurobiol 91:200–219. https://doi.org/10.1016/j.pneurobio.2010.01.012
Yang CS, Wang H (2016) Cancer preventive activities of tea catechins. Molecules 21:1679. https://doi.org/10.3390/molecules21121679
Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP (1998) Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev 7:351–354
Zaveri NT (2006) Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci 78:2073–2080. https://doi.org/10.1016/j.lfs.2005.12.006
Acknowledgements
The authors thank Shu Chen and Mizuki Tsunakawa for their valuable discussions on this study and their technical assistance with the experiments. They also thank Masanobu Hibi and Koichi Misawa for proofreading this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
TH designed the study, conducted the experiments, and wrote the draft of the manuscript with support from YM and NO. TH and YM contributed to the analysis and interpretation of data with support from NO. KK designed and manufactured the test beverage and supported the design of the human study. TH revised the manuscript with support from YM. All the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
TH, KK, YM, and NO are employees of Kao Corporation, a chemical, cosmetic, and food company headquartered in Tokyo, Japan. Kao Corporation had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethics approval
The experimental protocol was approved by the Human Research Ethics Committee, Kao Corporation (Approval No. T153–180720, dated October 15, 2018).
Informed consent
Written informed consent was obtained from all participants prior to enrollment in accordance with the Declaration of Helsinki.
Additional information
Communicated by Michalis G Nikolaidis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasumura, T., Kinoshita, K., Minegishi, Y. et al. Combination of tea catechins and ornithine effectively activates the urea cycle: an in vitro and human pilot study. Eur J Appl Physiol 124, 827–836 (2024). https://doi.org/10.1007/s00421-023-05310-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05310-4