Abstract
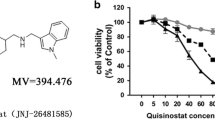
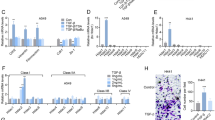
Histone deacetylase (HDAC) inhibitors have a potential therapeutic role for non-small cell lung cancer (NSCLC). However, more preclinical studies of HDAC inhibitors in NSCLC and normal lung epithelial cells are required to evaluate their antitumor activities and mechanisms. The bicellular tight junction molecule claudin-2 (CLDN-2) is highly expressed in lung adenocarcinoma tissues and increase the proliferation of adenocarcinoma cells. Downregulation of the tricellular tight junction molecule angulin-1/LSR induces malignancy via EGF-dependent CLDN-2 and TGF-β-dependent cellular metabolism in human lung adenocarcinoma cells. In the present study, to investigate the detailed mechanisms of the antitumor activities of HDAC inhibitors in lung adenocarcinoma, human lung adenocarcinoma A549 cells and normal lung epithelial cells were treated with the HDAC inibitors Trichostatin A (TSA) and Quisinostat (JNJ-2648158) with or without TGF-β. Both HDAC inhibitors increased anguin-1/LSR, decrease CLDN-2, promoted G1 arrest and prevented the migration of A549 cells. Furthermore, TSA but not Quisinostat with or without TGF-β induced cellular metabolism indicated as the mitochondrial respiration measured using the oxygen consumption rate. In normal human lung epithelial cells, treatment with TSA and Quisinostat increased expression of LSR and CLDN-2 and decreased that of CLDN-1 with or without TGF-β in 2D culture. Quisinostat but not TSA with TGF-β increased CLDN-7 expression in 2D culture. Both HDAC inhibitors prevented disruption of the epithelial barrier measured as the permeability of FD-4 induced by TGF-β in 2.5D culture. TSA and Quisinostat have potential for use in therapy for lung adenocarcinoma via changes in the expression of angulin-1/LSR and CLDN-2.







Similar content being viewed by others
References
Akizuki R, Eguchi H, Endo S, Matsunaga T, Ikari A (2019) ZO-2 suppresses cell migration mediated by a reduction in matrix metalloproteinase 2 in claudin-18-expressing lung adenocarcinoma A549 cells. Biol Pharm Bull 42:247–254
Arai W, Konno T, Kohno T, Kodera Y, Tsujiwaki M, Shindo Y, Chiba H, Miyajima M, Sakuma Y, Watanabe A, Kojima T (2020) Downregulation of angulin-1/LSR induces malignancy via upregulation of EGF-dependent claudin-2 and TGF-β-dependent cell metabolism in human lung adenocarcinoma A549 cells. Oncotarget (in press)
Arts J, King P, Mariën A, Floren W, Beliën A, Janssen L, Pilatte I, Roux B, Decrane L, Gilissen R, Hickson I, Vreys V, Cox E, Bol K, Talloen W, Goris I, Andries L, Du Jardin M, Janicot M, Page M, van Emelen K, Angibaud P (2009) JNJ-26481585, a novel “second-generation” oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin Cancer Res 15:6841–6851
Bao L, Diao H, Dong N, Su X, Wang B, Mo Q, Yu H, Wang X, Chen C (2016) Histone deacetylase inhibitor induces cell apoptosis and cycle arrest in lung cancer cells via mitochondrial injury and p53 up-acetylation. Cell Biol Toxicol 32:469–482
Bartling B, Hofmann HS, Boettger T, Hansen G, Burdach S, Silber RE, Simm A (2005) Comparative application of antibody and gene array for expression profiling in human squamous cell lung carcinoma. Lung Cancer 49:145–154
Benny S, Mishra R, Manojkumar MK, Aneesh TP (2020) From Warburg effect to Reverse Warburg effect; the new horizons of anti-cancer therapy. Med Hypotheses 144:110216
Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C (2017) Progress and prospects of early detection in lung cancer. Open Biol 7:170070
Chikamatsu K, Ishii H, Murata T, Sakakura K, Shino M, Toyoda M, Takahashi K, Masuyama K (2013) Alteration of cancer stem cell-like phenotype by histone deacetylase inhibitors in squamous cell carcinoma of the head and neck. Cancer Sci 104:1468–1475
Dhawan P, Ahmad R, Chaturvedi R, Smith JJ, Midha R, Mittal MK, Krishnan M, Chen X, Eschrich S, Yeatman TJ, Harris RC, Washington MK, Wilson KT, Beauchamp RD, Singh AB (2011) Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene 30:3234–3247
Fukuda K, Takeuchi S, Arai S, Katayama R, Nanjo S, Tanimoto A, Nishiyama A, Nakagawa T, Taniguchi H, Suzuki T, Yamada T, Nishihara H, Ninomiya H, Ishikawa Y, Baba S, Takeuchi K, Horiike A, Yanagitani N, Nishio M, Yano S (2019) Epithelial-to-mesenchymal transition is a mechanism of alk inhibitor resistance in lung cancer independent of ALK mutation status. Cancer Res 79:1658–1670
Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkühler C (2007) HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res 17:195–211
Hadnagy A, Beaulieu R, Balicki D (2008) Histone tail modifications and noncanonical functions of histones: perspectives in cancer epigenetics. Mol Cancer Ther 7:740–748
He B, Dai L, Zhang X, Chen D, Wu J, Feng X, Zhang Y, Xie H, Zhou L, Wu J, Zheng S (2018) The HDAC inhibitor quisinostat (JNJ-26481585) supresses hepatocellular carcinoma alone and synergistically in combination with sorafenib by G0/G1 phase arrest and apoptosis induction. Int J Biol Sci 14:1845–1858
Herbst RS, Morgensztern D, Boshoff C (2018) The biology and management of non-small cell lung cancer. Nature 553:446–454
Hichino A, Okamoto M, Taga S, Akizuki R, Endo S, Matsunaga T, Ikari A (2017) Down-regulation of claudin-2 expression and proliferation by epigenetic inhibitors in human lung adenocarcinoma A549 cells. J Biol Chem 292:2411–2421
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu YL, Paz-Ares L (2017) Lung cancer: current therapies and new targeted treatments. Lancet 389:299–311
Hua W, Ten Dijke P, Kostidis S, Giera M, Hornsveld M (2020) TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell Mol Life Sci 77:2103–2123
Hull EE, Montgomery MR, Leyva KJ (2016) HDAC inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. Biomed Res Int 2016:8797206
Ikari A, Sato T, Watanabe R, Yamazaki Y, Sugatani J (2012) Increase in claudin-2 expression by an EGFR/MEK/ERK/c-Fos pathway in lung adenocarcinoma A549 cells. Biochim Biophys Acta 1823:1110–1118
Jones PA, Issa JP, Baylin S (2016) Targeting the cancer epigenome for therapy. Nat Rev Genet 17:630–641
Kaarteenaho-Wiik R, Soini Y (2009) Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem 57:187–195
Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M (2008) Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J 409:581–589
Kodera Y, Chiba H, Konno T, Kohno T, Takahashi H, Kojima T (2020) HMGB1-downregulated angulin-1/LSR induces epithelial barrier disruption via claudin-2 and cellular metabolism via AMPK in airway epithelial Calu-3 cells. Biochem Biophys Res Commun 52:553–560
Koval M (2013) Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol 75:551–567
Kyuno T, Kyuno D, Kohno T, Konno T, Kikuchi S, Arimoto C, Yamaguchi H, Imamura M, Kimura Y, Kondoh M, Takemasa I, Kojima T (2020) Tricellular tight junction protein LSR/angulin-1 contributes to the epithelial barrier and malignancy in human pancreatic cancer cell line. Histochem Cell Biol 153:5–16
Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P (2015) Lung cancer: Biology and treatment options. Biochim Biophys Acta 1856:189–210
Li Y, Seto E (2016) HDACs and hdac inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med 6:a026831
Luo S, Ma K, Zhu H, Wang S, Liu M, Zhang W, Liang S, Xu N (2017) Molecular, biological characterization and drug sensitivity of chidamide-resistant non-small cell lung cancer cells. Oncol Lett 14:6869–6875
Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M (2011) LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci 124:548–555
Milazzo G, Mercatelli D, Di Muzio G, Triboli L, De Rosa P, Perini G, Giorgi FM (2020) Histone deacetylases (HDACs): evolution, specificity, role in transcriptional complexes, and pharmacological actionability. Genes (Basel) 11:556
Mottamal M, Zheng S, Huang TL, Wang G (2015) Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules 20:3898–3941
Mukhopadhyay NK, Weisberg E, Gilchrist D, Bueno R, Sugarbaker DJ, Jaklitsch MT (2006) Effectiveness of trichostatin A as a potential candidate for anticancer therapy in non-small-cell lung cancer. Ann Thorac Surg 81:1034–1042
Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM (2001) The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res 61:8492–8497
Ohta H, Chiba S, Ebina M, Furuse M, Nukiwa T (2012) Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol 302:L193–L205
Okada T, Konno T, Kohno T, Shimada H, Saito K, Satohisa S, Saito T, Kojima T (2020) Possibility of targeting claudin-2 in therapy for human endometrioid endometrial carcinoma. Reprod Sci 27:2092–2103
Overgaard CE, Mitchell LA, Koval M (2012) Roles for claudins in alveolar epithelial barrier function. Ann N Y Acad Sci 1257:167–174
Pavlova NN, Thompson CB (2016) The emerging hallmarks of cancer metabolism. Cell Metab 23:27–47
Runkle EA, Mu D (2013) Tight junction proteins: from barrier to tumorigenesis. Cancer Lett 337:41–48
Shimada H, Satohisa S, Kohno T, Takahashi S, Hatakeyama T, Konno T, Tsujiwaki M, Saito T, Kojima T (2016) The roles of tricellular tight junction protein lipolysis-stimulated lipoprotein receptor in malignancy of human endometrial cancer cells. Oncotarget 7:27735–27752
Shimada H, Satohisa S, Kohno T, Konno T, Takano KI, Takahashi S, Hatakeyama T, Arimoto C, Saito T, Kojima T (2017a) Downregulation of lipolysis-stimulated lipoprotein receptor promotes cell invasion via claudin-1-mediated matrix metalloproteinases in human endometrial cancer. Oncol Lett 14:6776–6782
Shimada H, Abe S, Kohno T, Satohisa S, Konno T, Takahashi S, Hatakeyama T, Arimoto C, Kakuki T, Kaneko Y, Takano KI, Saito T, Kojima T (2017b) Loss of tricellular tight junction protein LSR promotes cell invasion and migration via upregulation of TEAD1/AREG in human endometrial cancer. Sci Rep 7:37049. https://doi.org/10.1038/srep37049
Singh AB, Sharma A, Dhawan P (2010) Claudin family of proteins and cancer: an overview. J Oncol 2010:541957
Song X, Wu JQ, Yu XF, Yang XS, Yang Y (2018) Trichostatin A inhibits proliferation of triple negative breast cancer cells by inducing cell cycle arrest and apoptosis. Neoplasma 65:898–906
Takano K, Kakuki T, Obata K, Nomura K, Miyata R, Kondo A, Kurose M, Kakiuchi A, Kaneko Y, Kohno T, Himi T, Kojima T (2016) The behavior and role of lipolysis-stimulated lipoprotein receptor, a component of tricellular tight junctions, in head and neck squamous cell carcinomas. Anticancer Res 36:5895–5904
Togami K, Yamaguchi K, Chono S, Tada H (2017) Evaluation of permeability alteration and epithelial-mesenchymal transition induced by transforming growth factor-β1 in A549, NCI-H441, and Calu-3 cells: development of an in vitro model of respiratory epithelial cells in idiopathic pulmonary fibrosis. J Pharmacol Toxicol Methods 86:19–27
Uchibori K, Satouchi M, Sueoka-Aragane N, Urata Y, Sato A, Imamura F, Inoue T, Tachihara M, Kobayashi K, Katakami N, Kokan C, Hirashima T, Iwanaga K, Mori M, Aoe K, Morita S, Negoro (2018) Phase II trial of gefitinib plus pemetrexed after relapse using first-line gefitinib in patients with non-small cell lung cancer harboring EGFR gene mutations. Lung Cancer 124:65–70
Varadarajan S, Stephenson RE, Miller AL (2019) Multiscale dynamics of tight junction remodeling. J Cell Sci 132:jcs229286
Ververis K, Hiong A, Karagiannis TC, Licciardi PV (2013) Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biologics 7:47–60
Wang X, Xu J, Wang H, Wu L, Yuan W, Du J, Cai S (2015) Trichostatin A, a histone deacetylase inhibitor, reverses epithelial-mesenchymal transition in colorectal cancer SW480 and prostate cancer PC3 cells. Biochem Biophys Res Commun 456:320–326
Wang SC, Wang ST, Liu HT, Wang XY, Wu SC, Chen LC, Liu YW (2017) Trichostatin A induces bladder cancer cell death via intrinsic apoptosis at the early phase and Sp1-survivin downregulation at the late phase of treatment. Oncol Rep 38:1587–1596
Yoon S, Eom GH (2016) HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J 52:1–11
Yoshida GJ (2015) Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res 34:111
Zhao R, Chen K, Cao J, Yu H, Tian L, Liu M (2016) A correlation analysis between HDAC1 over-expression and clinical features of laryngeal squamous cell carcinoma. Acta Otolaryngol 136:172–176
Zihni C, Mills C, Matter K, Balda MS (2016) Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 17:564–580
Acknowledgements
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labour and Welfare of Japan (T. Kojima:19K07464; W. Arai:20K17186) and by a grant-in-aid from GlaxoSmithKline Japan (T. Konno).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shindo, Y., Arai, W., Konno, T. et al. Effects of histone deacetylase inhibitors Tricostatin A and Quisinostat on tight junction proteins of human lung adenocarcinoma A549 cells and normal lung epithelial cells. Histochem Cell Biol 155, 637–653 (2021). https://doi.org/10.1007/s00418-021-01966-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-021-01966-1