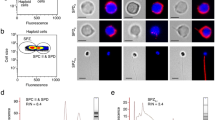
Abstract
Much progress has been made regarding our understanding of aromatase regulation, estrogen synthesis partitioning and communication between the germinal and somatic compartments of the differentiating gonad. We now know that most of the enzymatic and signaling apparatus required for steroidogenesis is endogenously expressed within germ cells. However, less is known about the expression and localization of steroidogenic components within mature spermatozoa. We have assembled a sperm library presenting 197,015 putative transcripts. Co-expression clustering analysis revealed that 6687 genes were present at higher levels in sperm in comparison to fifteen other salmon tissue libraries. The sperm transcriptome is highly complex containing the highest proportion of unannotated genes (45%) of the tissues analyzed. Our analysis of highly expressed genes in late-stage sperm revealed dedication to tasks involving chromatin remodeling, flagellogenesis and proteolysis. In addition, using various different embedding and microscopic techniques, we examined the morphology of salmon spermatozoa and characterized expression and localization of several estrogenic regulatory and signaling proteins by immunohistochemistry. We provide evidence for the endogenous synthesis and localization of aromatase (CYP19A and CYP19B1) and potential mediators of estrogen [i.e., ER-alpha and soluble adenylyl cyclase (sAC)] or phosphate (i.e., CREB and FOXL2A) signaling. Partitioning of select transcripts that encode AR-beta, FSH and the LH receptor, but not AR-alpha, LH or the FSH receptor, further points to localized specificity of function in the steroidogenic circuitry of the sperm cell. These results open new avenues of investigation to further our understanding of the intra- and intercellular regulatory processes that guide sperm development and biology.










Similar content being viewed by others
Abbreviations
- AR:
-
Androgen receptor (α and β)
- cAMP:
-
cyclic AMP
- CREB:
-
cAMP response element binding protein
- CREM:
-
cAMP response element modulator
- CYP19A and CYP19B:
-
Cytochrome P450 superfamily aromatase A and B
- DAX-1:
-
Dosage-sensitive sex reversal, adrenal hypoplasia congenital, critical region on the X-chromosome, gene-1
- DM-domain proteins:
-
Doublesex- and male abnormal-3 (MAB-3)-related transcription (DMRT) factors
- E2:
-
17-β-estradiol
- ER:
-
Estrogen receptor
- FOXL2:
-
Forkhead box L2
- IHC:
-
Immunohistochemistry
- MBM:
-
Plastic, methyl methacrylate/butyl methacrylate
- MRPL4:
-
Mitochondrial riboprotein L4
- MRPS14:
-
Mitochondrial riboprotein S14
- MIS:
-
Müllerian inhibiting substance (also known as anti-Müllerian hormone or AMH)
- ODF:
-
Outer dense fibers
- PDE:
-
Phosphodiesterase
- PKA:
-
Protein kinase A
- RPL26:
-
Cytoplasmic riboprotein L26
- RPS29:
-
Cytoplasmic riboprotein S29
- sAC:
-
Soluble adenylyl cyclase
- TUBA:
-
α-Tubulin
References
Aquila S, Sisci D, Gentile M, Middea E, Siciliano L, Andò S (2002) Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab 87:3385–3390
Aquila S, Sisci D, Gentile M, Middea E, Catalano S, Carpino A, Rago V, Andò S (2004) Estrogen receptor (ER)α and ERβ are both expressed in human ejaculated spermatozoa: evidence of their direct interaction with phosphatidylinositol-3-OH kinase/Akt pathway. J Clin Endocrinol Metab 89:1443–1451
Beltrán C, Vacquier VD, Moy G, Chen Y, Buck J, Levin LR, Darszon A (2007) Particulate and soluble adenylyl cyclases participate in the sperm acrosome reaction. Biochem Biophys Res Commun 358:1128–1135
Cappallo-Obermann H, Schulze W, Jastrow H, Baukloh V, Spiess A-N (2011) Highly purified spermatozoal RNA obtained by a novel method indicates an unusual 28S/18S rRNA ratio and suggests impaired ribosome assembly. Mol Hum Reprod 17:669–678
Caulier M, Brion F, Chadili E, Turies C, Piccini B, Porcher J-M, Guiguen Y, Hinfray N (2015) Localization of steroidogenic enzymes and Foxl2a in the gonads of mature zebrafish (Danio rerio). Comp Biochem Physiol A Mol Integr Physiol 188:96–106
Chen J-Q, Yager JD, Russo J (2005) Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta 1746:1–17
Chennathukuzhi V, Morales CR, El-Alfy M, Hecht NB (2003) The kinesin KIF17b and RNA-binding protein TB-RBP transport specific cAMP-responsive element modulator-regulated mRNAs in male germ cells. Proc Natl Acad Sci USA 100:15566–15571
Delalande C, Goupil A-S, Lareyre J-J, Le Gac F (2015) Differential expression patterns of three aromatase genes and of four estrogen receptors genes in the testes of trout (Oncorhynchus mykiss). Mol Reprod Dev 82:694–708
Dı́ez-Sánchez C, Ruiz-Pesini E, Montoya J, Pérez-Martos A, Enrı́quez JA, López-Pérez MJ (2003) Mitochondria from ejaculated human spermatozoa do not synthesize proteins. FEBS Lett 553:205–208
Eddy EM, Toshimori K, O’Brien DA (2003) Fibrous sheath of mammalian spermatozoa. Microsc Res Tech 61:103–115
Fang P, Zeng P, Wang Z, Liu M, Xu W, Dai J, Zhao X, Zhang D, Liang D, Chen X, Shi S, Zhang M, Wang L, Qiao Z, Shi H (2014) Estimated diversity of messenger RNAs in each murine spermatozoa and their potential function during early zygotic development. Biol Reprod 90(5):94–111
Garden G, Canady K, Lurie D, Bothwell M, Rubel E (1994) A biphasic change in ribosomal conformation during transneuronal degeneration is altered by inhibition of mitochondrial, but not cytoplasmic protein synthesis. J Neurosci 14:1994–2008
Gohin M, Bodinier P, Fostier A, Chesnel F, Bobe J (2011) Aromatase is expressed and active in the rainbow trout oocyte during final oocyte maturation. Mol Reprod Dev 78:510–518
Guiguen Y, Fostier A, Piferrer F, Chang C-F (2010) Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol 165:352–366
Gur Y, Breitbart H (2008) Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Mol Cell Endocrinol 282:45–55
Hammes SR, Levin ER (2007) Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741
Haramoto Y, Takahashi S, Asashima M (2006) Two distinct domains in pro-region of Nodal-related 3 are essential for BMP inhibition. Biochem Biophys Res Commun 346:470–478
Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB (2005) The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9:249–259
Holdcraft RW, Braun RE (2004) Hormonal regulation of spermatogenesis. Int J Androl 27:335–342
Inaba K (2003) Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool Sci 20:1043–1056
Ingraham HA, Hirokawa Y, Roberts LM, Mellon SH, McGee E, Nachtigal MW, Visser JA (2000) Autocrine and paracrine Müllerian inhibiting substance hormone signaling in reproduction. Recent Prog Horm Res 55:53–67
Johnson GD, Sendler E, Lalancette C, Hauser R, Diamond MP, Krawetz SA (2011) Cleavage of rRNA ensures translational cessation in sperm at fertilization. Mol Hum Reprod 17:721–726
Johnson GD, Mackie P, Jodar M, Moskovtsev S, Krawetz SA (2015) Chromatin and extracellular vesicle associated sperm RNAs. Nucleic Acids Res 43:6847–6859
Kikuchi K, Hamaguchi S (2013) Novel sex-determining genes in fish and sex chromosome evolution. Dev Dyn 242:339–353
Kimmins S, Kotaja N, Davidson I, Sassone-Corsi P (2004) Testis-specific transcription mechanisms promoting male germ-cell differentiation. Reproduction 128:5–12
Kleene KC (2013) Connecting cis-elements and trans-factors with mechanisms of developmental regulation of mRNA translation in meiotic and haploid mammalian spermatogenic cells. Reproduction 146:R1–R19
Kotaja N, Sassone-Corsi P (2007) The chromatoid body: a germ-cell-specific RNA-processing centre. Nat Rev Mol Cell Biol 8:85–90
Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, Draghici S, Diamond MP (2011) A survey of small RNAs in human sperm. Hum Reprod 26:3401–3412
Krisfalusi M, Miki K, Magyar PL, O’Brien DA (2006) Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod 75:270–278
Kumar RS, Trant JM (2001) Piscine glycoprotein hormone (gonadotropin and thyrotropin) receptors: a review of recent developments. Comp Biochem Physiol B Biochem Mol Biol 129:347–355
Liu Y, Dettin LE, Folmer J, Zirkin BR, Papadopoulos V (2007) Abnormal morphology of spermatozoa in cytochrome P450 17alpha-hydroxylase/17,20-lyase (CYP17) deficient mice. J Androl 28:453–460
Manandhar G, Schatten H, Sutovsky P (2005) Centrosome reduction during gametogenesis and its significance. Biol Reprod 72:2–13
Mao S, Goodrich RJ, Hauser R, Schrader SM, Chen Z, Krawetz SA (2013) Evaluation of the effectiveness of semen storage and sperm purification methods for spermatozoa transcript profiling. Syst Biol Reprod Med 59:287–295
McDonald KL (1994) Electron microscopy and EM immunocytochemistry. Methods Cell Biol 44:411–444
McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL (2008) The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biol Reprod 79:889–896
Mizrahi R, Breitbart H (2014) Mitochondrial PKA mediates sperm motility. Biochim Biophys Acta 1840:3404–3412
Morisawa M, Ishida K (1987) Short-term changes in levels of cyclic AMP, adenylate cyclase, and phosphodiesterase during the initiation of sperm motility in rainbow trout. J Exp Zool 242:199–204
Morisawa S, Morisawa M (1988) Induction of potential for sperm motility by bicarbonate and pH in rainbow trout and chum salmon. J Exp Biol 136:13–22
Nagler JJ, Cavileer T, Sullivan J, Cyr DG, Rexroad C (2007) The complete nuclear estrogen receptor family in the rainbow trout: discovery of the novel ERα2 and both ERβ isoforms. Gene 392:164–173
Noda T, Shidara O, Harayama H (2012) Detection of the activator cAMP responsive element modulator (CREM) isoform ortholog proteins in porcine spermatids and sperm. Theriogenology 77:1360–1368
Pedram A, Razandi M, Deschenes RJ, Levin ER (2012) DHHC-7 and -21 are palmitoyl acyltransferases for sex steroid receptors. Mol Biol Cell 23:188–199
Pisarska MD, Kuo FT, Bentsi-Barnes IK, Khan S, Barlow GM (2010) LATS1 phosphorylates forkhead L2 and regulates its transcriptional activity. Am J Physiol Endocrinol Metab 299:E101–E109
Pisarska MD, Barlow G, Kuo F-T (2011) Minireview: roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology. Endocrinology 152:1199–1208
Sarkar S, Jun S, Simpkins JW (2015) Estrogen amelioration of Aβ-induced defects in mitochondria is mediated by mitochondrial signaling pathway involving ERβ, AKAP and Drp1. Brain Res 1616:101–111
Schatten H, Sun Q-Y (2010) The role of centrosomes in fertilization, cell division and establishment of asymmetry during embryo development. Semin Cell Dev Biol 21:174–184
Schulz RW, de França LR, Lareyre J-J, LeGac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R, Krawetz SA (2013) Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res 41:4104–4117
Shiraishi E, Yoshinaga N, Miura T, Yokoi H, Wakamatsu Y, Abe S-I, Kitano T (2008) Müllerian inhibiting substance is required for germ cell proliferation during early gonadal differentiation in medaka (Oryzias latipes). Endocrinology 149:1813–1819
Skepper JN (2000) Immunocytochemical strategies for electron microscopy: choice or compromise. J Microsc 199:1–36
Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE (2005) Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Hum Reprod 20:3481–3487
Tresguerres M, Barott KL, Barron ME, Roa JN (2014) Established and potential physiological roles of bicarbonate-sensing soluble adenylyl cyclase (sAC) in aquatic animals. J Exp Biol 217:663–672
Turner RM (2003) Tales from the tail: what do we really know about sperm motility? J Androl 24:790–803
Vadnais ML, Cao W, Aghajanian HK, Haig-Ladewig L, Lin AM, Al-Alao O, Gerton GL (2014) Adenine nucleotide metabolism and a role for AMP in modulating flagellar waveforms in mouse sperm. Biol Reprod 90(6):128. doi:10.1095/biolreprod.113.114447
Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G (2013) cAMP and mitochondria. Physiology 28:199–209
Viñas J, Piferrer F (2008) Stage-specific gene expression during fish spermatogenesis as determined by laser-capture microdissection and quantitative-PCR in sea bass (Dicentrarchus labrax) gonads. Biol Reprod 79:738–747
von Schalburg KR, Rise ML, Brown GD, Davidson WS, Koop BF (2005) A comprehensive survey of the genes involved in maturation and development of the rainbow trout ovary. Biol Reprod 72:687–699
von Schalburg KR, Yasuike M, Yazawa R, de Boer JG, Reid L, So S, Robb A, Rondeau EB, Phillips RB, Davidson WS, Koop BF (2011) Regulation and expression of sexual differentiation factors in embryonic and extragonadal tissues of Atlantic salmon. BMC Genomics 12:31–49
von Schalburg KR, Gowen BE, Rondeau EB, Johnson NW, Minkley DR, Leong JS, Davidson WS, Koop BF (2013) Sex-specific expression, synthesis and localization of aromatase regulators in one-year-old Atlantic salmon ovaries and testes. Comp Biochem Physiol B Biochem Mol Biol 164:236–246
von Schalburg KR, Gowen BE, Messmer AM, Davidson WS, Koop BF (2014) Sex-specific expression and localization of aromatase and its regulators during embryonic and larval development of Atlantic salmon. Comp Biochem Physiol B Biochem Mol Biol 168:33–44
Acknowledgements
We appreciate very much the assistance of Tim Hewison (Grieg Seafood B.C. Ltd., Campbell River, B.C.) and Lance Page (Marine Harvest Canada, Duncan, B.C.) for their provision of the testis and sperm samples, respectively. We also would like to thank Dr. Duncan Liew (EMD Millipore Corp.) and Dr. Sybille Rex (Abcam Incorp.) for their help in the selection of commercial antibodies compatible with a salmon study. We are grateful to James Nagler (University of Idaho, Moscow, ID) and Martin Tresguerres (University of California, San Diego, CA), for generously providing antibodies to trout ERα and sAC, respectively. The contribution of ribosomes from Bruno Klaholz (CBI-IGBMC, Illkirch, France) was invaluable to our control experiments for Y10b labeling. We thank Patrick Nahirney (University of Victoria, Faculty of Medicine) for access to his Jeol TEM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The animals used in this study underwent normal commercial broodstock practices (sperm retrieval) or destined for food production (testis extraction).
Funding
This research was supported by a Natural Resources and Applied Sciences Team Grant from the B.C. Innovation Council (WSD, BFK) and the Natural Sciences and Engineering Research Council of Canada (BFK, WSD).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
von Schalburg, K.R., Gowen, B.E., Leong, J.S. et al. Subcellular localization and characterization of estrogenic pathway regulators and mediators in Atlantic salmon spermatozoal cells. Histochem Cell Biol 149, 75–96 (2018). https://doi.org/10.1007/s00418-017-1611-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-017-1611-3