Abstract
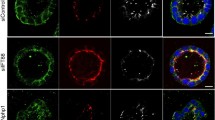
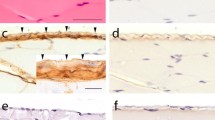
The amount of hyaluronan (HA) is low in simple epithelia under normal conditions, but during tumorigenesis, trauma or inflammation HA is increased on the epithelial cells and surrounding stroma. Excessive HA in epithelia is suggested to interfere with cell–cell adhesions, resulting in disruption of the epithelial barrier function. In addition, stimulated HA synthesis has been correlated with epithelial-to-mesenchymal transition and invasion of cancer cells. However, the effects of HA overload on normal epithelial morphogenesis have not been characterized in detail. Madin-Darby canine kidney (MDCK) cells form polarized epithelial cysts, when grown in a 3-dimensional (3D) matrix. These cells were used to investigate whether stimulated HA synthesis, induced by stable overexpression of GFP-HAS3, influences cell polarization and epithelial morphogenesis. GFP-HAS3 expression in polarized MDCK cells resulted in active HA secretion at apical and basolateral membrane domains. HA-deposits interfered with the formation of cell–cell junctions, resulting in impaired barrier function. In 3D cyst cultures, HA accumulated into apical lumina and was also secreted from the basal side. The HAS3-expressing cysts failed to form a single lumen and instead displayed multiple small lumina. This phenotype was correlated with aberrant mitotic spindle orientation in dividing cells. The results of this study indicate that excess pericellular HA disturbs the normal cell–cell and cell–ECM interactions in simple epithelia, leading to aberrant epithelial morphogenesis. The morphological abnormalities observed in 3D epithelial cultures upon stimulated HAS3 expression may be related to premalignant changes, including intraluminal invasion and deregulated epithelialization, probably mediated by the mitotic spindle orientation defects.






Similar content being viewed by others
Abbreviations
- bHABC:
-
Biotinylated hyaluronan binding complex
- ELSA:
-
Enzyme-linked sorbent assay
- fHABC:
-
Fluorescent hyaluronan binding complex
- FBS:
-
Fetal bovine serum
- GFP:
-
Green fluorescent protein
- HA:
-
Hyaluronan
- HAS:
-
Hyaluronan synthase
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
References
Anttila MA, Tammi RH, Tammi MI, Syrjänen KJ, Saarikoski SV, Kosma VM (2000) High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res 60:150–155
Asselman M, Verhulst A, De Broe ME, Verkoelen CF (2003) Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol 14:3155–3166
Asselman M, Verhulst A, Van Ballegooijen ES, Bangma CH, Verkoelen CF, De Broe ME (2005) Hyaluronan is apically secreted and expressed by proliferating or regenerating renal tubular cells. Kidney Int 68:71–83
Auvinen P, Tammi R, Parkkinen J, Tammi M, Ågren U, Johansson R, Hirvikoski P, Eskelinen M, Kosma VM (2000) Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 156:529–536
Brecht M, Mayer U, Schlosser E, Prehm P (1986) Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem J 239:445–450
Bryant DM, Mostov KE (2008) From cells to organs: building polarized tissue. Natl Rev Mol Cell Biol 9:887–901
Chow G, Tauler J, Mulshine JL (2010) Cytokines and growth factors stimulate hyaluronan production: role of hyaluronan in epithelial to mesenchymal-like transition in non-small cell lung cancer. J Biomed Biotechnol 2010:485468
deS Senanayake P, Calabro A, Nishiyama K, Hu JG, Bok D, Hollyfield JG (2001) Glycosaminoglycan synthesis and secretion by the retinal pigment epithelium: polarized delivery of hyaluronan from the apical surface. J Cell Sci 114:199–205
Evanko SP, Angello JC, Wight TN (1999) Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19:1004–1013
Fujiwara T, Kawakatsu T, Tayama S, Kobayashi Y, Sugiura N, Kimata K, Takai Y (2008) Hyaluronan-CD44 pathway regulates orientation of mitotic spindle in normal epithelial cells. Genes Cells 13:759–770
Goransson V, Johnsson C, Jacobson A, Heldin P, Hallgren R, Hansell P (2004) Renal hyaluronan accumulation and hyaluronan synthase expression after ischaemia-reperfusion injury in the rat. Nephrol Dial Transplant 19:823–830
Gumbiner BM (2005) Regulation of cadherin-mediated adhesion in morphogenesis. Natl Rev Mol Cell Biol 6:622–634
Gumbiner B, Simons K (1986) A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol 102:457–468
Hiltunen EL, Anttila M, Kultti A, Ropponen K, Penttinen J, Yliskoski M, Kuronen AT, Juhola M, Tammi R, Tammi M, Kosma VM (2002) Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Res 62:6410–6413
Itano N, Atsumi F, Sawai T, Yamada Y, Miyaishi O, Senga T, Hamaguchi M, Kimata K (2002) Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci USA 99:3314–3609
Jaffe AB, Kaji N, Durgan J, Hall A (2008) Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 183:625–633
Karvinen S, Pasonen-Seppänen S, Hyttinen JM, Pienimäki JP, Törrönen K, Jokela TA, Tammi MI, Tammi R (2003) Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J Biol Chem 278:49495–49504
Kass L, Erler JT, Dembo M, Weaver VM (2007) Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol 39:1987–1994
Knudson CB (2003) Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res C Embryo Today 69:174–196
Kosaki R, Watanabe K, Yamaguchi Y (1999) Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res 59:1141–1145
Kultti A, Rilla K, Tiihonen R, Spicer AP, Tammi RH, Tammi MI (2006) Hyaluronan synthesis induces microvillus-like cell surface protrusions. J Biol Chem 281:15821–15828
Lechler T, Fuchs E (2005) Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437:275–280
Li Y, Heldin P (2001) Hyaluronan production increases the malignant properties of mesothelioma cells. Br J Cancer 85:600–667
Lubarsky B, Krasnow MA (2003) Tube morphogenesis: making and shaping biological tubes. Cell 112:19–28
Marieb EA, Zoltan-Jones A, Li R, Misra S, Ghatak S, Cao J, Zucker S, Toole BP (2004) Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res 64:1229–1232
Myllymäki SM, Teräväinen TP, Manninen A (2011) Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PLoS One 6:e19453
Neame SJ, Isacke CM (1993) The cytoplasmic tail of CD44 is required for basolateral localization in epithelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. J Cell Biol 121:1299–1310
Pasonen-Seppänen S, Karvinen S, Törrönen K, Hyttinen JM, Jokela T, Lammi MJ, Tammi MI, Tammi R (2003) EGF upregulates, whereas TGF-beta downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J Invest Dermatol 120:1038–1044
Prehm P (1983) Synthesis of hyaluronate in differentiated teratocarcinoma cells. Characterization of the synthase. Biochem J 211:181–189
Reinsch S, Karsenti E (1994) Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J Cell Biol 126:1509–1526
Rilla K, Siiskonen H, Spicer AP, Hyttinen JM, Tammi MI, Tammi RH (2005) Plasma membrane residence of hyaluronan synthase is coupled to its enzymatic activity. J Biol Chem 280:31890–31897
Rilla K, Tiihonen R, Kultti A, Tammi M, Tammi R (2008) Pericellular hyaluronan coat visualized in live cells with a fluorescent probe is scaffolded by plasma membrane protrusions. J Histochem Cytochem 56:901–910
Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Ågren U, Alhava E, Kosma VM (1998) Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 58:342–347
Schepers MS, Asselman M, Duim RA, Romijn JC, Schroder FH, Verkoelen CF (2003) Pericellular matrix formation by renal tubule epithelial cells in relation to crystal binding. Nephron Exp Nephrol 94:e103–e112
Siiskonen H, Torronen K, Kumlin T, Rilla K, Tammi MI, Tammi RH (2011) Chronic UVR causes increased immunostaining of CD44 and accumulation of hyaluronan in mouse epidermis. J Histochem Cytochem 59:908–917
Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM (2011a) Hyaluronan in human malignancies. Exp Cell Res 317:383–391
Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM (2011b) Hyaluronan in human malignancies. Exp Cell Res 317:383–391
Tammi RH, Tammi MI (2004) Hyaluronan in the epidermis and other epithelial tissues. In: Garg HG, Hales CA (eds) Chemistry and biology of hyaluronan. Elsevier, Amsterdam
Tammi RH, Tammi MI (2009) Hyaluronan accumulation in wounded epidermis: a mediator of keratinocyte activation. J Invest Dermatol 129:1858–1860
Tammi R, Ripellino JA, Margolis RU, Maibach HI, Tammi M (1989) Hyaluronate accumulation in human epidermis treated with retinoic acid in skin organ culture. J Invest Dermatol 92:326–332
Tammi R, Säämänen AM, Maibach HI, Tammi M (1991) Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J Invest Dermatol 97:126–130
Tammi R, Rilla K, Pienimäki JP, MacCallum DK, Hogg M, Luukkonen M, Hascall VC, Tammi M (2001) Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem 276:35111–35122
Tammi R, Pasonen-Seppänen S, Kolehmainen E, Tammi M (2005) Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol 124:898–905
Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI (2008a) Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol 18:288–295
Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI (2008b) Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol 18:288–295
Toyoshima F, Nishida E (2007) Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J 26:1487–1498
Vigetti D, Genasetti A, Karousou E, Viola M, Moretto P, Clerici M, Deleonibus S, De Luca G, Hascall VC, Passi A (2010) Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor-kappaB (NF-kappaB) pathway. J Biol Chem 285:24639–24645
Wang C, Tammi M, Tammi R (1992) Distribution of hyaluronan and its CD44 receptor in the epithelia of human skin appendages. Histochemistry 98:105–112
Weigel PH, DeAngelis PL (2007) Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem 282:36777–36781
Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM (2005) Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell 16:433–445
Zegers MM, O’Brien LE, Yu W, Datta A, Mostov KE (2003) Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol 13:169–176
Zoltan-Jones A, Huang L, Ghatak S, Toole BP (2003) Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem 278:45801–45810
Acknowledgments
Special thanks are due to Laura Halttunen, Heli Huttunen, Eija Kettunen and Eija Rahunen for expert technical assistance. We also want to thank Satu Myllymäki for valuable help in mitotic spindle orientation analysis. This work was supported by the Academy of Finland, grants #131771 (S P–S.), #114330𡆈 (A.M.) and #107173 (M.T.) and by Sigrid Juselius Foundation (A.M., R.T. and M.T.) and the special funds (Kärkihanke) allocated by the University of Eastern Finland to the Cancer Center of Eastern Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rilla, K., Pasonen-Seppänen, S., Kärnä, R. et al. HAS3-induced accumulation of hyaluronan in 3D MDCK cultures results in mitotic spindle misorientation and disturbed organization of epithelium. Histochem Cell Biol 137, 153–164 (2012). https://doi.org/10.1007/s00418-011-0896-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-011-0896-x