Abstract
Purpose
Williams-Beuren syndrome (WBS) is a rare genetic disease characterized by psychomotor delay, cardiovascular, musculoskeletal, and endocrine problems. Retinal involvement, which is not well characterized, has also been described. The purpose of this cross-sectional study is to describe the characteristics in optical coherence tomography (OCT) and OCT-angiography (OCTA) of patients with WBS.
Methods
We included patients with WBS confirmed by genetic analysis. The patients underwent OCT (30° × 25°, 61 B-scans) and OCTA (10° × 10° and 20° × 20°) examinations, all centered on the. Data on retinal thickness (total, inner and outer layers) and foveal morphology on OCT and vessel and perfusion density in OCTA (VD and PD, respectively) were collected. These data were compared with an age-matched control group.
Results
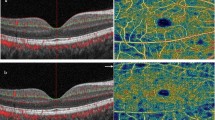
22 eyes of 22 patients with WBS (10 females, mean age 31.5 years) were included. Retinal thickness (and specifically inner retinal layers) in OCT was significantly reduced in all sectors (central, parafoveal, and perifoveal) compared to the control group (p < 0.001 in all sectors). Fovea in WBS eyes was broader and shallower than controls. The PD and VD in both 10 and 20 degrees of fields in OCTA was significantly reduced in patients with WBS, in all vascular plexa (all p < 0.001).
Conclusions
This study is the first to quantify and demonstrate retinal structural and microvascular alterations in patients with WBS. Further studies with longitudinal data will reveal the potential clinical relevance of these alterations.



Similar content being viewed by others
References
Williams JC, Barratt-Boyes BG, Lowe JB (1961) Supravalvular aortic stenosis. Circulation 24:1311–1318. https://doi.org/10.1161/01.cir.24.6.1311
Beuren AJ, Apitz J, Harmjanz D (1962) Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation 26:1235–1240. https://doi.org/10.1161/01.cir.26.6.1235
Pober BR (2010) Williams-Beuren syndrome. N Engl J Med 362:239–252. https://doi.org/10.1056/NEJMra0903074
Kozel BA, Barak B, Kim CA et al (2021) Williams syndrome. Nat Rev Dis Primers 7:42. https://doi.org/10.1038/s41572-021-00276-z
Greenberg F, Lewis RA (1988) The Williams syndrome. Spectrum and significance of ocular features. Ophthalmology 95:1608–1612. https://doi.org/10.1016/s0161-6420(88)32959-3
Winter M, Pankau R, Amm M et al (1996) The spectrum of ocular features in the Williams-Beuren syndrome. Clin Genet 49:28–31. https://doi.org/10.1111/j.1399-0004.1996.tb04320.x
Huryn LA, Flaherty T, Nolen R, et al. (2022) Novel ophthalmic findings and deep phenotyping in Williams-Beuren syndrome. Br J Ophthalmol bjophthalmol-2022–321103. https://doi.org/10.1136/bjophthalmol-2022-321103
Di Marino M, Cesareo M, Aloe G et al (2020) Retinal and Choroidal Vasculature in Patients with Marfan Syndrome. Transl Vis Sci Technol 9:5. https://doi.org/10.1167/tvst.9.9.5
Chen H, Ng KY, Li S et al (2022) Characteristics of the foveal microvasculature in children with marfan syndrome: An Optical Coherence Tomography Angiography Study. Retina 42:138–151. https://doi.org/10.1097/IAE.0000000000003272
Ghoraba HH, Moshfeghi DM (2023) Retinal arterial tortuosity in Ehlers-Danlos syndromes. Eye (Lond) 37:1936–1941. https://doi.org/10.1038/s41433-022-02278-x
Bedeschi MF, Bianchi V, Colli AM et al (2011) Clinical follow-up of young adults affected by Williams syndrome: experience of 45 Italian patients. Am J Med Genet A 155A:353–359. https://doi.org/10.1002/ajmg.a.33819
Zhang C, Tatham AJ, Weinreb RN et al (2014) Relationship between ganglion cell layer thickness and estimated retinal ganglion cell counts in the glaucomatous macula. Ophthalmology 121:2371–2379. https://doi.org/10.1016/j.ophtha.2014.06.047
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300:5–25. https://doi.org/10.1002/cne.903000103
Campbell JP, Zhang M, Hwang TS et al (2017) Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep 7:42201. https://doi.org/10.1038/srep42201
Chanwimol K, Balasubramanian S, Nassisi M et al (2019) Retinal Vascular Changes During Pregnancy Detected With Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 60:2726–2732. https://doi.org/10.1167/iovs.19-26956
Castelo-Branco M, Mendes M, Sebastião AR et al (2007) Visual phenotype in Williams-Beuren syndrome challenges magnocellular theories explaining human neurodevelopmental visual cortical disorders. J Clin Invest 117:3720–3729. https://doi.org/10.1172/JCI32556
Tick S, Rossant F, Ghorbel I et al (2011) Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci 52:5105–5110. https://doi.org/10.1167/iovs.10-7005
Vajzovic L, Hendrickson AE, O’Connell RV et al (2012) Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol 154:779-789.e2. https://doi.org/10.1016/j.ajo.2012.05.004
McCafferty BK, Wilk MA, McAllister JT et al (2015) Clinical Insights Into Foveal Morphology in Albinism. J Pediatr Ophthalmol Strabismus 52:167–172. https://doi.org/10.3928/01913913-20150427-06
Fieß A, Pfisterer A, Gißler S et al (2022) Retinal thickness and foveal hypoplasia in adults born preterm with and without retinopathy of prematurity: The Gutenberg Prematurity Eye Study. Retina 42:1716–1728. https://doi.org/10.1097/IAE.0000000000003501
Lavia C, Bonnin S, Maule M et al (2019) Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina 39:247–258. https://doi.org/10.1097/IAE.0000000000002413
Lavia C, Mecê P, Nassisi M et al (2020) Retinal Capillary Plexus Pattern and Density from Fovea to Periphery Measured in Healthy Eyes with Swept-Source Optical Coherence Tomography Angiography. Sci Rep 10:1474. https://doi.org/10.1038/s41598-020-58359-y
Rogaczewska M, Michalak S, Stopa M (2021) Macular vessel density differs in multiple sclerosis and neuromyelitis optica spectrum disorder: An optical coherence tomography angiography study. PLoS One 16:e0253417. https://doi.org/10.1371/journal.pone.0253417
Hansen C, Bojikian KD, Chu Z et al (2020) Macular microvascular parameters in the ganglion cell-inner plexiform layer derived by optical coherence tomography angiography: Vascular structure-central visual function analysis. PLoS One 15:e0240111. https://doi.org/10.1371/journal.pone.0240111
López-Cuenca I, Salobrar-García E, Elvira-Hurtado L et al (2021) The Value of OCT and OCTA as Potential Biomarkers for Preclinical Alzheimer’s Disease: A Review Study. Life (Basel) 11:712. https://doi.org/10.3390/life11070712
Hohberger B, Lucio M, Schlick S et al (2021) OCT-angiography: Regional reduced macula microcirculation in ocular hypertensive and pre-perimetric glaucoma patients. PLoS One 16:e0246469. https://doi.org/10.1371/journal.pone.0246469
Pröschel C, Blouin MJ, Gutowski NJ et al (1995) Limk1 is predominantly expressed in neural tissues and phosphorylates serine, threonine and tyrosine residues in vitro. Oncogene 11:1271–1281
Gray V, Karmiloff-Smith A, Funnell E, Tassabehji M (2006) In-depth analysis of spatial cognition in Williams syndrome: A critical assessment of the role of the LIMK1 gene. Neuropsychologia 44:679–685. https://doi.org/10.1016/j.neuropsychologia.2005.08.007
Hirota H, Matsuoka R, Chen X-N et al (2003) Williams syndrome deficits in visual spatial processing linked to GTF2IRD1 and GTF2I on chromosome 7q11.23. Genet Med 5:311–321. https://doi.org/10.1097/01.GIM.0000076975.10224.67
Edelmann L, Prosnitz A, Pardo S et al (2007) An atypical deletion of the Williams-Beuren syndrome interval implicates genes associated with defective visuospatial processing and autism. J Med Genet 44:136–143. https://doi.org/10.1136/jmg.2006.044537
Bayarsaihan D, Bitchevaia N, Enkhmandakh B et al (2003) Expression of BEN, a member of TFII-I family of transcription factors, during mouse pre- and postimplantation development. Gene Expr Patterns 3:579–589. https://doi.org/10.1016/s1567-133x(03)00118-2
Morris CA, Mervis CB, Hobart HH et al (2003) GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet A 123A:45–59. https://doi.org/10.1002/ajmg.a.20496
Chen K, Weiland JD (2014) Discovery of retinal elastin and its possible role in age-related macular degeneration. Ann Biomed Eng 42:678–684. https://doi.org/10.1007/s10439-013-0936-x
Boned-Murillo A, Albertos-Arranz H, Diaz-Barreda MD et al (2021) Optical Coherence Tomography Angiography in Diabetic Patients: A Systematic Review. Biomedicines 10:88. https://doi.org/10.3390/biomedicines10010088
Remolí Sargues L, Monferrer Adsuara C, Castro Navarro V, et al. (2022) Swept-source optical coherence tomography angiography automatic analysis of microvascular changes secondary to systemic hypertension. Eur J Ophthalmol 11206721221146674. https://doi.org/10.1177/11206721221146674
Sadun AA, Win PH, Ross-Cisneros FN et al (2000) Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 98:223–232 discussion 232-235
Carelli V, La Morgia C, Valentino ML et al (2009) Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim Biophys Acta 1787:518–528. https://doi.org/10.1016/j.bbabio.2009.02.024
Kang EY-C, Liu P-K, Wen Y-T et al (2021) Role of Oxidative Stress in Ocular Diseases Associated with Retinal Ganglion Cells Degeneration. Antioxidants (Basel) 10:1948. https://doi.org/10.3390/antiox10121948
Ferrari M, Stagi S (2021) Oxidative Stress in Down and Williams-Beuren Syndromes: An Overview. Molecules 26:3139. https://doi.org/10.3390/molecules26113139
Acknowledgements
This work has been generated within the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability (ERN-ITHACA) (EU Framework Partnership Agreement ID: 3HP-HP-FPA ERN-01-2016/739516).
Funding
Partial financial support was received from the Italian Ministry of Health.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Marco Nassisi, Claudia Mainetti, Andrea Sperti, Guido Galmozzi, Andrea Aretti, Gaia Leone, Valeria Nicotra, Federico Grilli and Berardo Rinaldi. Federica Natacci, Maria Francesca Bedeschi and Francesco Viola supervised the study. The first draft of the manuscript was written by Marco Nassisi and Claudia Mainetti and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the local ethics committee (Comitato Etico Milano Area 2, protocol n.1179 of the 29th of April 2022).
Informed consent
Informed consent was obtained from all individual participants or legal guardians after explanation of the study and its potential outcomes.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nassisi, M., Mainetti, C., Sperti, A. et al. Optical coherence tomography angiography findings in Williams-Beuren syndrome. Graefes Arch Clin Exp Ophthalmol 262, 1131–1140 (2024). https://doi.org/10.1007/s00417-023-06323-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06323-7