Abstract
Purpose
To evaluate the effect of low-dose atropine eyedrops on pupil metrics.
Methods
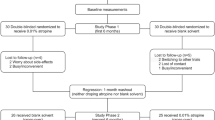
This study was based on a randomized, double-masked, placebo-controlled, and cross-over trial in mainland China. In phase 1, subjects received 0.01% atropine or placebo once nightly. After 1 year, the atropine group switched to placebo (atropine-placebo group), and the placebo group switched to atropine (placebo-atropine group). Ocular parameters were measured at the crossover time point (at the 12th month) and the 18th month.
Results
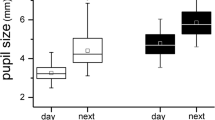
Of 105 subjects who completed the study, 48 and 57 children were allocated into the atropine-placebo and placebo-atropine groups, respectively. After cessation, the photopic pupil diameter (PD) and mesopic PD both decreased (− 0.46 ± 0.47 mm, P < 0.001; − 0.30 ± 0.74 mm, P = 0.008), and the constriction ratio (CR, %) increased (4.39 ± 7.54, P < 0.001) compared with values at the crossover time point of the atropine-placebo group; pupil metrics of the atropine-placebo group had no difference from the values at the crossover time point of the placebo-atropine group. After 6 months of treatment, the photopic PD and the mesopic PD increased (0.54 ± 0.67 mm, P < 0.001; 0.53 ± 0.89 mm, P < 0.001), the CR (%) decreased (− 2.53 ± 8.64, P < 0.001) compared with values at the crossover time point of the placebo-atropine group. There was no significant relationship between pupil metrics and myopia progression during 0.01% atropine treatment.
Conclusion
Pupil metrics and the CR could return to pre-atropine levels after cessation. Pupil metrics had no significant effect on myopia progression during treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
The increasing prevalence of myopia and high myopia has brought significant economic and social burdens [1, 2]. At present, clinical guidelines for myopia control include orthokeratology lenses, contact lenses with peripheral defocus design, maximizing time spent outdoors, and low-concentration atropine eyedrops [3,4,5,6,7,8]. Studies have shown that low-concentration atropine eyedrops are one of the most effective treatments for myopia [8,9,10]. However, the ideal atropine concentration has yet to be determined since the higher the concentration of atropine eyedrops, the better the effect of controlling myopia development, but with more apparent adverse effects and more obvious rebound after drug cessation [11,12,13].
The main side effects of atropine eyedrops are photophobia, glare, and near blur [9, 14, 15]. The ocular symptoms may be related to mydriasis and impaired pupillary light reflex (PLR) [16]. Differences in atropine concentration, race, and follow-up durations among studies may have contributed to different proportions of photophobic glare and near-blurred vision [17]. There were relatively limited studies reporting changes in pupil metrics after the use of atropine [12, 18, 19]. Fu et al. reported that 0.02% and 0.01% atropine increased pupil diameter (PD) similarly after 4 months (0.87 mm) and 12 months (0.77 mm; P = 0.55) of treatment [19]. Yam and colleagues showed that the increase in pupil size followed a concentration-related response [20]. In addition, Chen et al. suggested that a larger PD induced a higher intensity of myopic shift in the peripheral retina, exerting a more significant suppressive effect on axial growth [21]. As the assessment of pupil appearance and PLR may inform us of the integrity of the autonomic nervous system, and measures of pupillary metrics are safe and noninvasive to characterize the mechanism of drug action, it is necessary to monitor changes in pupil metrics during atropine treatment and after cessation [22, 23].
In this study, children who had used 0.01% atropine eyedrops for 1 year were followed up for another 6 months after drug cessation. In addition, we also evaluated the change of pupil metrics of children using 0.01% atropine during the same period and explored whether pupil metrics played a role in controlling myopia progression during atropine treatment.
Methods
Study design and setting
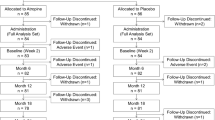
This study was based on a randomized, double-masked, placebo-controlled, and cross-over trial which comprised 2 phases in mainland China. The detailed design and methods have been described previously in phase 1 [8]. Briefly, children aged 6 to 12 years old with spherical equivalent (SE) refraction range of − 1.00 to − 6.00 D in both eyes, astigmatism of less than 1.50 D in both eyes, and intraocular pressure of less than 21 mmHg were enrolled in this study. In phase 1 (the first year), 220 subjects were randomized to receive either 0.01% placebo or atropine eye drops at bedtime every night in both eyes for 1 year. In phase 2 (the second year), the placebo group was crossed over to the 0.01% atropine group (referred to as the “placebo-atropine group”), and the 0.01% atropine group was crossed over to the placebo group (referred to as the “atropine-placebo group”) for 1 year. All eye drops were prepared in mono-dose preparation by Shenyang Xingqi Pharmaceutical Co, Ltd (Shenyang, PR. China). Our study reported the results from the crossover time point (at the 12th month) to the 18th month.
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Tongren Hospital. All participants provided written informed consent after agreeing to enrollment. The trial was registered on the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx). The registration number is ChiCTR-IOR-17013898 [24].
Outcome measurements
From the crossover time point (at the 12th month) to the 18th month, subjects underwent the same standardized ophthalmic examinations as in phase 1. Measurements were taken from 9:00 to 12:00 at the weekend. The measurement of pupil sizes was examined before measuring the axial length (AL) and refractive error. The OPD-Scan III (Nidek, Japan) was applied to measure mesopic and photopic pupil sizes. The protocol parameters of the device were reset before each measurement. We had patients sit directly across from the examiner; participants were asked to fixate on a distant object to relax their accommodation with the left eye that was not being measured. Then, the mesopic (background intensity was 0 μw) pupil size was measured three times and averaged using the OPD-Scan III, followed by photopic illuminance (background intensity as 50 μw). For each set of measurements, the average value of the first three consecutive data captures with differences less than 0.50 mm was used for analyses. We assessed the following parameters using the following equations [25]:
Statistical analysis
Statistical analyses were performed using commercial software (SPSS version 25.0; SPSS, Inc., Chicago, IL, US). Mean values for ocular parameters were calculated from the right eyes. Categorical data were represented as counts (frequencies). Mean ± standard deviation values were used to describe continuous variables. The Kolmogorov–Smirnov test was used to examine the distributions of continuous data. Continuous data with normal distributions were analyzed with paired T-tests within the group. Continuous variables with abnormal distributions were analyzed with Mann–Whitney U-tests or Wilcoxon rank-sum tests. The chi-square test was used to assess the difference in gender between the two groups. The change of parameters was defined by the difference between the crossover time point (at the 12th month) and the corresponding follow-up values.
To explore whether the pupil metrics can recover after cessation, an independent-sample T-test was used to compare pupillary parameters of the atropine-placebo group (values at the 18th month) with the placebo-atropine group (values at the 12th month).
The multivariable regression model was conducted to investigate whether pupil metrics contributed to myopia progression during atropine treatment. Univariable analysis was also performed to assess the associated factors for myopia progression. Multivariable analysis was performed using variables with P values less than 0.2 in univariable analysis. A P value < 0.05 with two-sided was considered statistically significant.
Results
In this study, one hundred and five (47.73%) children with available data were enrolled. Forty-eight subjects were in the atropine-placebo group and fifty-seven were in the placebo-atropine group. No significant difference was found between the demographic characteristics of the atropine-placebo group and the placebo-atropine group at the crossover time point (Table 1).
For the atropine-placebo group, 6 months after treatment cessation, the photopic PD and mesopic PD decreased significantly compared with the end of atropine treatment (3.86 ± 0.55 mm vs. 3.40 ± 0.42 mm, P < 0.001; 5.91 ± 0.58 mm vs. 5.61 ± 0.65 mm, P = 0.008); the constriction ratio (%) increased significantly (34.73 ± 5.45 mm vs. 39.12 ± 6.58 mm, P < 0.001; Tables 2, 3). For the placebo-atropine group, 6 months after atropine treatment, the increase of photopic PD and mesopic PD was significant (3.36 ± 0.46 mm vs. 3.90 ± 0.61 mm, P < 0.001; 5.50 ± 0.75 mm vs. 6.04 ± 0.65 mm, P < 0.001) and the constriction ratio decreased (38.67 ± 6.34 vs. 36.14 ± 6.62, P = 0.03) compared to pre-atropine screening (at the 12th month). The PLR of atropine-placebo group and placebo-atropine group changed slightly (2.05 ± 0.34 mm vs. 2.21 ± 0.51 mm, P = 0.07; 2.15 ± 0.53 mm vs. 2.17 ± 0.47 mm, P = 0.81; Tables 2, 3).
The change in pupil metrics, including photopic PD, mesopic PD, and constriction ratio, differed between the two groups (P < 0.001), while the change in PLR showed no significant difference (P = 0.27, Table 3). Six months after cessation, pupil metrics of the atropine-placebo group had no difference from the values of the placebo-atropine group at the 12th month (Table 4).
Table 5 shows the association between myopia progression and pupil metrics using univariable analysis. The univariable analysis showed that the change of SE was related to age at myopia onset (P < 0.2; β, 0.19), time spent on near work (P < 0.2; β, 0.21), time outdoors (P < 0.05; β, 0.38), constriction ratio at the crossover time point (P < 0.02; β, − 0.21), change of photopic PD (P < 0.2; β, − 0.20). To eliminate nonsignificant factors, we conducted multivariable linear regression analysis. The change of SE was no longer associated with age at myopia onset (P = 0.57; β, 0.09), time spent on near work (P = 0.43; β, 0.12), time outdoors (P = 0.05; β, 0.32), constriction ratio at the crossover time point (P = 0.99; β, − 0.0036) or change of photopic PD (P = 0.45; β, − 0.17, Table 5).
The univariable analysis showed that the change of AL was related to time spent on near work (P < 0.2; β, 0.23), change of photopic PD (P < 0.2; β, 0.20), change of CR (%) (P < 0.2; β, − 0.23). We conducted multivariable linear regression analysis to eliminate nonsignificant factors. The change of photopic PD (P = 0.32; β, 0.18) and change of CR (%) (P = 0.57; β, − 0.10) was no longer associated with the change of AL (Table 5); while, the time spent on near work (P < 0.05; β, 0.31) was still related to the change of AL.
Discussion
This study found that pupil size and the constriction ratio could return to pre-atropine levels after cessation. A once-nightly dose of 0.01% atropine eyedrops induced the PD increase and decreased constriction ratio but did not influence the PLR. The condition of pupil metrics before atropine treatment and the changes of pupillary parameters during treatment had no significant effect on myopia progression.
As a nonselective muscarinic antagonist agent, low-dose atropine for controlling myopia progression has aroused general interest, and its efficacy has been preliminarily recognized in recent years [12]. The eyedrops block both the pupillary sphincter and ciliary muscle, causing its main ocular symptom of photophobia [26, 27]. In clinical trials of different concentrations of atropine, some subjects dropped out due to the side effect [28,29,30].
Studies have reported that children in 0.01% and 0.02% atropine groups were photophobic in bright sunlight at the beginning of the treatment, but the symptom was not obvious in normal indoor lighting or when the sunlight was not intense outside [31]. Most subjects could adjust to photophobia caused by slightly dilated pupils after a period of atropine treatment [31].
According to Yam’s report, after 4 months of 0.01% atropine eyedrops treatment, photopic PD increased by 0.26 ± 0.83 mm and mesopic PD increased by 0.18 ± 0.46 mm; after 8 months of treatment, photopic PD increased by 0.41 ± 0.80 mm and mesopic PD increased by 0.16 ± 0.46 mm [10]. In our study, the increase of photopic and mesopic PD was 0.54 ± 0.67 mm and 0.53 ± 0.89 mm. It should be noted that the increase in PD in our study was larger than that in Yam’s, which may be related to the different periods. Another study that used 0.01% atropine eyedrops found that photopic PD increased by 0.77 mm after the 4-month treatment and 0.74 mm after the 8-month treatment (pupil measurement was under 300 to 310 lx illumination) [19]. The variation was a bit larger than ours. The difference may be related to the mode of the instrument measurement and the background light intensity set. And the effect of atropine varies with race-related melanin levels within the iris [16].
Most studies reported the psychophysical changes 1 year after the instillation of the drops. In a meta-analysis, 1-year randomized controlled trials showed both photopic (weighted mean difference, 0.35 mm; 95% CI = 0.02, 0.68) and mesopic PD (weighted mean difference, 0.51 mm; 95% CI = 0.31, 0.71) increased significantly in the 0.01% atropine group compared with control groups [11]. As previous studies reported, subjects using 0.01% atropine for 1 year showed an increase in photopic PD (ranging from 0.26 to 1.2 mm), and mesopic PD (ranging from 0.09 to 1.15 mm) (Table 6) [9, 10, 19, 32, 33]. Cooper et al. proposed that when pupil dilation exceeds 3 mm, noticeable photophobia could appear in daily visual tasks [16]. In Cooper’s clinical trial, among the 0.025%, 0.05%, 0.08%, 0.125%, 0.166%, 0.225%, 0.333%, 0.40%, and 0.50% atropine, 0.02% atropine was the highest concentration which did not result in clinical symptoms [16]. The pupil dilation of our placebo-atropine group was below the threshold of no more than 3 mm.
The Atropine Treatment of Myopia trials (ATOM 2) phase 2 study assessed changes in pupil size in eyes treated with 0.01%, 0.1%, and 0.5% atropine after cessation [34]. After cessation of atropine, mesopic and photopic pupil sizes in all groups reduced continuously in the following 12 months. Eight months after cessation, the pupil sizes were slightly smaller than in the first screening visit in all three groups [34]. Zhu et al. also reported that pupil size returned to pre-atropine levels at the end of follow-up in Chinese children [35]. Our study also showed a recovery of pupil metrics after the cessation of treatment. Thus, adverse effects of atropine could be eliminated by a gradual cessation and elimination of atropine.
Some studies indicated that the change of PD after atropine treatment may contribute to myopic progression. Fu et al. reported that the AL of children with a smaller PD might increase rapidly while receiving atropine treatment, indicating that changes in PD may suggest the response to the effects of low-concentration atropine [36]. It also suggested that girls with slower myopia progression reported more photophobia issues than girls with a higher progression rate [37]. Consistent conclusions have been made in orthokeratology lens treatment trials. Larger pupil diameters facilitated the effect of the orthokeratology lens to slow axial growth in myopia [21, 38].
Some mechanisms have been postulated to explain the association between myopia and pupil metrics. Pupil size could determine the amount of light entering the eyes, and larger pupils could allow more light to reach the peripheral retina, resulting in more peripheral defocus and thus affecting myopic progression [21, 39]. Wong et al. found that PD after 20 min of dark adaptation in the early-onset myopes was 4.52 mm, which was significantly smaller than that in the emmetropes (5.21 mm) (P < 0.05) [40]. They speculated that small PD may play an essential role in the pathogenesis of early-onset myopia via its effect on the depth of focus [40]. However, our study did not find the impact of pupillary parameters on the myopic progression of 0.01% atropine, which may be due to the small sample size and short period. Further research is still needed to illustrate whether the pupil size of participants could affect myopia control efficacy among interventions.
Strength and limitation
This study was based on a randomized, double-blind trial and continued following up on pupil metrics during and after atropine treatment. There are some limitations of the present study. First, an acknowledged weakness of the study was the lack of baseline measurement of pupil metrics for the atropine-placebo group before atropine treatment. We planned to measure the pupil parameters of the subjects in the initial protocol. Unfortunately, since the measuring instrument needed to be purchased from abroad, the study had been conducted for 1 year when the instrument arrived at the experimental site. Regrettably, we failed to measure the pupil metrics at the beginning of the first year. Since the placebo-atropine group served as a blank control in the first year, the pupil was not affected by the drug, we used the pupil metrics of the placebo-atropine group before atropine treatment as a comparison group. These two groups were matched for baseline characteristics, so the direct comparison between the two groups was deemed appropriate. Second, the dropout rate was high because of the pandemic sparked by the Covid-19, whereas there was no statistical difference between lost-to-follow and continuing participants. More extensive studies with different atropine concentrations and longer follow-ups may help validate the specific long-term effects of atropine on pupil metrics.
References
Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith Iii EL, Zhou X, Matsui KO, Wu PC, Sankaridurg P, Chia A, Rosman M, Lamoureux EL, Man R, He M (2020) Myopia Nature Rev Disease Prim 6:99. https://doi.org/10.1038/s41572-020-00231-4
Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123:1036–1042. https://doi.org/10.1016/j.ophtha.2016.01.006
Chen X, Xiong Y, Liu F, Wang J, Yang B, Liu L (2022) Factors determining the myopia control effect of an orthokeratology lens: a two-year multi-level model. Ophthalmic & physiological optics: J Br College Ophthalmic Optic (Optometrists). https://doi.org/10.1111/opo.12990
Fricke TR, Sankaridurg P, Naduvilath T, Resnikoff S, Tahhan N, He M, Frick KD (2022) Establishing a method to estimate the effect of antimyopia management options on lifetime cost of myopia. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2021-320318
Gispets J, Yébana P, Lupón N, Cardona G, Pérez-Corral J, Pauné J, Cortilla B (2022) Efficacy, predictability and safety of long-term orthokeratology: an 18-year follow-up study. Cont Lens Anterior Eye: J Br Contact Lens Assoc 45:101530. https://doi.org/10.1016/j.clae.2021.101530
Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, Logan NS, Liu M, Morgan I, Ohno-Matsui K, Pärssinen O, Resnikoff S, Sankaridurg P, Saw SM, Smith EL 3rd, Tan DTH, Walline JJ, Wildsoet CF, Wu PC, Zhu X, Wolffsohn JS (2021) IMI Prevention of Myopia and its progression. Invest Ophthalmol Vis Sci 62:6. https://doi.org/10.1167/iovs.62.5.6
Li SM, Li H, Li SY, Liu LR, Kang MT, Wang YP, Zhang F, Zhan SY, Gopinath B, Mitchell P, Wang N (2015) Time outdoors and myopia progression over 2 years in Chinese children: the Anyang childhood eye study. Invest Ophthalmol Vis Sci 56:4734–4740. https://doi.org/10.1167/iovs.14-15474
Wei S, Li S-M, An W, Du J, Liang X, Sun Y, Zhang D, Tian J, Wang N (2020) Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2020.3820
Chia A, Chua W-H, Cheung Y-B, Wong W-L, Lingham A, Fong A, Tan D (2012) Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology 119:347–354. https://doi.org/10.1016/j.ophtha.2011.07.031
Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP (2019) Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 126:113–124. https://doi.org/10.1016/j.ophtha.2018.05.029
Tsai HR, Chen TL, Wang JH, Huang HK, Chiu CJ (2021) Is 0.01% Atropine an effective and safe treatment for myopic children? a systemic review and meta-analysis. J Clin Med 10. https://doi.org/10.3390/jcm10173766
Chia A, Lu QS, Tan D (2016) Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology 123:391–399. https://doi.org/10.1016/j.ophtha.2015.07.004
Pérez-Flores I, Macías-Murelaga B, Barrio-Barrio J (2021) A multicenter Spanish study of atropine 0 01% in childhood myopia progression. Sci Rep 11:21748. https://doi.org/10.1038/s41598-021-00923-1
Larkin GL, Tahir A, Epley KD, Beauchamp CL, Tong JT, Clark RA (2019) Atropine 0.01% eye drops for myopia control in American children: a multiethnic sample across three US sites. Ophthalmol Therapy 8:589–598. https://doi.org/10.1007/s40123-019-00217-w
Loughman J, Flitcroft DI (2016) The acceptability and visual impact of 0.01% atropine in a Caucasian population. Br J Ophthalmol 100:1525–1529. https://doi.org/10.1136/bjophthalmol-2015-307861
Cooper J, Eisenberg N, Schulman E, Wang FM (2013) Maximum atropine dose without clinical signs or symptoms. Optom Vis Sci 90:1467–1472. https://doi.org/10.1097/opx.0000000000000037
Ha A, Kim SJ, Shim SR, Kim YK, Jung JH (2022) Efficacy and safety of 8 atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology 129:322–333. https://doi.org/10.1016/j.ophtha.2021.10.016
Yam JC, Zhang XJ, Zhang Y, Wang YM, Tang SM, Li FF, Kam KW, Ko ST, Yip BHK, Young AL, Tham CC, Chen LJ, Pang CP (2022) Three-year clinical trial of low-concentration atropine for myopia progression (LAMP) study: continued versus washout: phase 3 report. Ophthalmology 129:308–321. https://doi.org/10.1016/j.ophtha.2021.10.002
Fu A, Stapleton F, Wei L, Wang W, Zhao B, Watt K, Ji N, Lyu Y (2020) Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol 104:1535–1541. https://doi.org/10.1136/bjophthalmol-2019-315440
Yam JC, Li FF, Zhang X, Tang SM, Yip BHK, Kam KW, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP (2020) Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study: phase 2 report. Ophthalmology 127:910–919. https://doi.org/10.1016/j.ophtha.2019.12.011
Chen Z, Niu L, Xue F, Qu X, Zhou Z, Zhou X, Chu R (2012) Impact of pupil diameter on axial growth in orthokeratology. Optom Vis Sci 89:1636–1640. https://doi.org/10.1097/OPX.0b013e31826c1831
Fosnaugh JS, Bunker EB, Pickworth WB (1992) Daily variation and effects of ambient light and circadian factors on the human light reflex. Methods Find Exp Clin Pharmacol 14:545–553
Chang LY-L, Turuwhenua J, Qu TY, Black JM, Acosta ML (2017) Infrared video pupillography coupled with smart phone LED for measurement of pupillary light reflex. Front Integr Neurosci 11:6. https://doi.org/10.3389/fnint.2017.00006
Wei S, Li SM, An W, Du J, Liang X, Sun Y, Zhang D, Tian J, Wang N (2020) Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol 138:1178–1184. https://doi.org/10.1001/jamaophthalmol.2020.3820
Chang DS, Arora K, Boland MV, Friedman DS (2019) The relationship between quantitative pupillometry and estimated ganglion cell counts in patients with glaucoma. J Glaucoma 28:238–242. https://doi.org/10.1097/ijg.0000000000001183
Kaymak H, Fricke A, Mauritz Y, Löwinger A, Klabe K, Breyer D, Lagenbucher A, Seitz B, Schaeffel F (2018) Short-term effects of low-concentration atropine eye drops on pupil size and accommodation in young adult subjects. Graefes Arch Clin Exp Ophthalmol 256:2211–2217. https://doi.org/10.1007/s00417-018-4112-8
Myles W, Dunlop C, McFadden SA (2021) The effect of long-term low-dose atropine on refractive progression in myopic Australian school children. J Clin Med 10. https://doi.org/10.3390/jcm10071444
Yen MY, Liu JH, Kao SC, Shiao CH (1989) Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol 21(5):180–2:187
Pineles SL, Kraker RT, VanderVeen DK, Hutchinson AK, Galvin JA, Wilson LB, Lambert SR (2017) Atropine for the prevention of myopia progression in children: a report by the American Academy of Ophthalmology. Ophthalmology 124:1857–1866. https://doi.org/10.1016/j.ophtha.2017.05.032
Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, Tan D (2006) Atropine for the treatment of childhood myopia. Ophthalmology 113:2285–2291. https://doi.org/10.1016/j.ophtha.2006.05.062
Cui C, Li X, Lyu Y, Wei L, Zhao B, Yu S, Rong J, Bai Y, Fu A (2021) Safety and efficacy of 0.02% and 0.01% atropine on controlling myopia progression: a 2-year clinical trial. Sci Rep 11:22267. https://doi.org/10.1038/s41598-021-01708-2
Hieda O, Hiraoka T, Fujikado T, Ishiko S, Hasebe S, Torii H, Takahashi H, Nakamura Y, Sotozono C, Oshika T, Morimoto T, Nishida K, Nishikawa N, Song YS, Tokutake T, Nishi Y, Shigeno Y, Kurihara T, Negishi K, Tsubota K, Ono M, Nakai T, Tan D, Tanaka S, Kinoshita S (2021) Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol 65:315–325. https://doi.org/10.1007/s10384-021-00822-y
Saxena R, Dhiman R, Gupta V, Kumar P, Matalia J, Roy L, Swaminathan M, Phuljhele S, Velpandian T, Sharma N (2021) Atropine for the treatment of childhood myopia in India: multicentric randomized trial. Ophthalmology 128:1367–1369. https://doi.org/10.1016/j.ophtha.2021.01.026
Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D (2014) Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol 157:451-457.e451. https://doi.org/10.1016/j.ajo.2013.09.020
Zhu Q, Tang Y, Guo L, Tighe S, Zhou Y, Zhang X, Zhang J, Zhu Y, Hu M (2020) Efficacy and safety of 1% atropine on retardation of moderate myopia progression in Chinese school children. Int J Med Sci 17:176–181. https://doi.org/10.7150/ijms.39365
Fu A, Stapleton F, Wei L, Wang W, Zhao B, Watt K, Yu S, Cui C, Lyu Y (2021) Risk factors for rapid axial length elongation with low concentration atropine for myopia control. Sci Rep 11:11729. https://doi.org/10.1038/s41598-021-88719-1
Michalski A, Rogaczewska M, Maleszka-Kurpiel M, Stopa M (2020) Pharmacological myopia control influence on quality of life and psyche among adolescents. J Clin Med 9. https://doi.org/10.3390/jcm9123920
Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R (2013) Factors preventing myopia progression with orthokeratology correction. Optom Vis Sci 90:1225–1236. https://doi.org/10.1097/OPX.0000000000000034
Zhou H, Zhao G, Li Y (2021) Adjunctive effects of orthokeratology and atropine 0.01% eye drops on slowing the progression of myopia. Clinic Exp Optom: 1–7. https://doi.org/10.1080/08164622.2021.1943318
Woung LC, Lue YF, Shih YF (1998) Accommodation and pupillary response in early-onset myopia among schoolchildren. Optom Vis Sci 75:611–616
Funding
This study was funded by grants from the Beijing Municipal Administration of Hospitals Incubating Program (PX2022007), the primary scientific research foundation for the junior researcher in Beijing Tongren Hospital, Capital Medical University (2020-YJJ-ZZL-011), the capital health research and development of special (2020–2-1081), the National Natural Science Foundation of China (82071000), and the Beijing Science Foundation for Distinguished Yong Scholars (JQ20029).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Ningli Wang and Shifei Wei.
(II) Administrative support: Ning-Li Wang.
(III) Provision of study materials or patients: Shifei Wei, Wei-Ling Bai, and Jia-He Gan.
(IV) Collection and assembly of data: Wei-Ling Bai, Jia-He Gan, and Shifei Wei.
(V) Data analysis and interpretation: Wei-Ling Bai.
(VI) Manuscript writing: All authors.
(VII) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board of Beijing Tongren Hospital, Capital Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The trial was registered on the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx). The registration number is ChiCTR-IOR-17013898.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bai, WL., Gan, JH., Wei, S. et al. Effect of low-dose atropine eyedrops on pupil metrics: results after half a year of treatment and cessation. Graefes Arch Clin Exp Ophthalmol 261, 1177–1186 (2023). https://doi.org/10.1007/s00417-022-05863-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05863-8