Abstract
Purpose
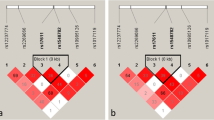
Age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV) are sister diseases and have several similar clinical features and still have few genetic differences. The association of HERPUD1 (homocysteine inducible ER protein with ubiquitin like domain 1) gene variant rs2217332 with PCV is known; however, such association with AMD has not been reported in the Indian population. We analyzed the association of rs2217332 with PCV and AMD to identify the preferential association of this variant with these diseases.
Methods
This is a population-based case–control study consisting of 422 patients (129 AMD cases; 101 PCV cases, 192 healthy controls) recruited from the vitreoretinal clinic Sankara Nethralaya. The sample size for the study was calculated using appropriate power calculation methods. Genotype was determined using PCR-based Sanger sequencing. The SPSS V23.0 statistical package tool was used to calculate chi-square and ROC to determine the association of rs2217332 with control, AMD, and PCV.
Results
Here, we report for the first time the association of this genetic variant (rs2217332) with AMD and PCV in the Indian population. The case–control study shows a significant association of this SNP with PCV (P value = 0.002); however, this variant is not significantly associated with AMD (P value = 0.602). Comparison between AMD (as control) and PCV (as case) also showed significant association of the SNP with PCV (P value = 0.02). Minor allele A conferred to increase the risk of PCV.
Conclusions
The study concludes that the genetic variant rs2217332 in HERPUD1 gene is highly significantly associated with PCV and not with AMD in Indian populations.


Similar content being viewed by others
References
Mitchell P, Liew G, Gopinath B, Wong TY (2018) Age-related macular degeneration. Lancet 392:1147–1159. https://doi.org/10.1016/S0140-6736(18)31550-2
Al-Zamil WM, Yassin SA (2017) Recent developments in age-related macular degeneration: a review. Clin Interv Aging 12:1313–1330. https://doi.org/10.2147/CIA.S143508
Algvere PV, Seregard S (2003) Drusen maculopathy: a risk factor for AMD. Can we prevent visual loss? Acta Ophthalmol Scand 81:427–429. https://doi.org/10.1034/j.1600-0420.2003.00157.x
Friedman E (1997) A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol 124:677–682. https://doi.org/10.1016/S0002-9394(14)70906-7
Stefánsson E, Geirsdóttir Á, Sigurdsson H (2011) Metabolic physiology in age related macular degeneration. Prog Retin Eye Res 30:72–80. https://doi.org/10.1016/j.preteyeres.2010.09.003
Biesemeier A, Taubitz T, Julien S et al (2014) Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging 35:2562–2573. https://doi.org/10.1016/j.neurobiolaging.2014.05.003
García-Layana A, Cabrera-López F, García-Arumí J et al (2017) Early and intermediate age-related macular degeneration: Update and clinical review. Clin Interv Aging 12:1579–1587. https://doi.org/10.2147/CIA.S142685
Kuo JZ, Wong TY, Ong FS (2013) Genetic risk, ethnic variations and pharmacogenetic biomarkers in age-related macular degeneration and polypoidal choroidal vasculopathy. Expert Rev Ophthalmol 8:127–140. https://doi.org/10.1586/eop.13.3
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B (1990) Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 10:1–8
Cheung CMG, Lai TYY, Ruamviboonsuk P et al (2018) Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology 125:708–724. https://doi.org/10.1016/j.ophtha.2017.11.019
Sahu Y, Chaudhary N, Joshi M, Gandhi A (2021) Idiopathic polypoidal choroidal vasculopathy: a review of literature with clinical update on current management practices. Int Ophthalmol 41:753–765. https://doi.org/10.1007/s10792-020-01620-0
Palkar AH, Khetan V (2019) Polypoidal choroidal vasculopathy: an update on current management and review of literature. Taiwan J Ophthalmol 9:72–92. https://doi.org/10.4103/tjo.tjo_35_18
Liu K, Chen LJ, Lai TYY et al (2014) Genes in the high-density lipoprotein metabolic pathway in age-related macular degeneration and polypoidal choroidal vasculopathy. Ophthalmology 121:911–916. https://doi.org/10.1016/j.ophtha.2013.10.042
Jin E, Bai Y, Huang L et al (2015) Evidence of a novel gene HERPUD1 in polypoidal choroidal vasculopathy. Int J Clin Exp Pathol 8:13928–13944
Alagappan LP, Koh JEW, Jahmunah V et al (2020) Development of an automated system for the detection of genotype in polypoidal choroidal vasculopathy using retinal image phenotype. Comput Methods Programs Biomed 192:105460. https://doi.org/10.1016/j.cmpb.2020.105460
Belal C, Ameli NJ, El Kommos A et al (2012) The homocysteine-inducible endoplasmic reticulum (ER) stress protein Herp counteracts mutant α-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins. Hum Mol Genet 21:963–977. https://doi.org/10.1093/HMG/DDR502
Cameron DJ, Galvin C, Alkam T et al (2012) Alzheimer’s-related peptide amyloid-β plays a conserved role in angiogenesis. PLoS One 7:e39598. https://doi.org/10.1371/journal.pone.0039598
Zhang X, Wen F, Zuo C et al (2011) Association of genetic variation on chromosome 9p21 with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 52:8063–8067. https://doi.org/10.1167/IOVS.11-7820
Cipriani V, Lorés-Motta L, He F et al (2020) Increased circulating levels of Factor H-Related Protein 4 are strongly associated with age-related macular degeneration. Nat Commun 11:778. https://doi.org/10.1038/s41467-020-14499-3
Yu Y, Bhangale TR, Fagerness J et al (2011) Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum Mol Genet 20:3699–3709. https://doi.org/10.1093/hmg/ddr270
Wen X, Liu Y, Yan Q et al (2018) Association of IGFN1 variant with polypoidal choroidal vasculopathy. J Gene Med 20:e3007. https://doi.org/10.1002/jgm.3007
Zhang X, Li M, Wen F et al (2013) Different impact of high-density lipoprotein-related genetic variants on polypoidal choroidal vasculopathy and neovascular age-related macular degeneration in a Chinese Han population. Exp Eye Res 108:16–22. https://doi.org/10.1016/J.EXER.2012.12.005
Tam PO, Ng TK, Liu DT et al (2008) HTRA1 variants in exudative age-related macular degeneration and interactions with smoking and CFH. Invest Ophthalmol Vis Sci 49(6):2357–65. https://doi.org/10.1167/iovs.07-1520
Liu K, Ma L, Lai TYY, Brelen ME, Tam POS, Tham CC, Pang CP, Chen LJ (2019) Evaluation of the association of C5 with neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Eye Vis (Lond) 6:34. https://doi.org/10.1186/s40662-019-0161-2
Ayub H, Shafique S, Azam A et al (2019) Association of rs10490924 in ARMS2/HTRA1 with age-related macular degeneration in the Pakistani population. Ann Hum Genet 83:285–290. https://doi.org/10.1111/ahg.12311
Chou Y-B, Hsu C-H, Chen W-S et al (2021) Deep learning and ensemble stacking technique for differentiating polypoidal choroidal vasculopathy from neovascular age-related macular degeneration. Sci Rep 11:7130. https://doi.org/10.1038/s41598-021-86526-2
Jin KW, Kim JH, Park JY et al (2021) Long-term outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Sci Rep 11:14623. https://doi.org/10.1038/s41598-021-93899-x
Azar G, Vasseur V, Lahoud C et al (2021) Polypoidal choroidal vasculopathy diagnosis and neovascular activity evaluation using optical coherence tomography angiography. Biomed Res Int 2021:1637377. https://doi.org/10.1155/2021/1637377
Gotoh N, Nakanishi H, Hayashi H et al (2009) ARMS2 (LOC387715) variants in Japanese patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Am J Ophthalmol 147(6):1037–41, 1041.e1–2. https://doi.org/10.1016/j.ajo.2008.12.036
Chantaren P, Ruamviboonsuk P, Ponglikitmongkol M et al (2012) Major single nucleotide polymorphisms in polypoidal choroidal vasculopathy: a comparative analysis between Thai and other Asian populations. Clin Ophthalmol 6:465–471. https://doi.org/10.2147/OPTH.S30529
Hayashi H, Yamashiro K, Gotoh N et al (2010) CFH and ARMS2 variations in age-related macular degeneration, polypoidal choroidal vasculopathy, and retinal angiomatous proliferation. Invest Ophthalmol Vis Sci 51:5914–5919. https://doi.org/10.1167/iovs.10-5554
Leveziel N, Zerbib J, Richard F et al (2008) Genotype-phenotype correlations for exudative age-related macular degeneration associated with homozygous HTRA1 and CFH genotypes. Invest Ophthalmol Vis Sci 49:3090–3094. https://doi.org/10.1167/iovs.07-1540
Tanaka K, Nakayama T, Mori R et al (2014) Associations of complement factor B and complement component 2 genotypes with subtypes of polypoidal choroidal vasculopathy. BMC Ophthalmol 14:83. https://doi.org/10.1186/1471-2415-14-83
Fritsche LG, Igl W, Bailey JNC et al (2016) A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet 48:134–143. https://doi.org/10.1038/ng.3448
Kondo N, Honda S, Ishibashi K et al (2008) Elastin gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 49:1101–1105. https://doi.org/10.1167/iovs.07-1145
Ma L, Liu K, Tsujikawa M et al (2016) Association of ABCG1 with neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in Chinese and Japanese. Invest Ophthalmol Vis Sci 57:5758–5763. https://doi.org/10.1167/iovs.16-20175
Hashikata H, Liu W, Inoue K et al (2010) Confirmation of an association of single-nucleotide polymorphism rs1333040 on 9p21 with familial and sporadic intracranial aneurysms in Japanese patients. Stroke 41:1138–1144. https://doi.org/10.1161/STROKEAHA.109.576694
Huang L, Zhang H, Cheng C-Y et al (2016) A missense variant in FGD6 confers increased risk of polypoidal choroidal vasculopathy. Nat Genet 48:640–647. https://doi.org/10.1038/ng.3546
Kumar M, Moptom SE, Sen P et al (2020) Prevalence of polypoidal choroidal vasculopathy in Indian population: risk factors, clinical and imaging characteristics. PLoS One 15:1–12. https://doi.org/10.1371/journal.pone.0231901
Funding
This work was supported by Bayer Zydus Pvt. Ltd, Bombay. Funding Reference Number: 35 (I) (II) 51 dated 13 November 2019.
Author information
Authors and Affiliations
Contributions
LPA and YR shared most of the experiments, performed the data analysis, and drafted the manuscript. DKS and JB performed some of the experiments. RR, PKS, SS, and MB facilitated the patient recruitment and reviewed the manuscript. SM developed the conceptual ideas and designed the study, drafted the manuscript, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Vision Research Foundation, Sankara Nethralaya Hospital Review Board (IRB Ethics Approval Number -Study code: 636–2017-P) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Name of the institute (IRB) that approved the study: Vision Research Foundation, Sankara Nethralya Hospital, Chennai.
Informed consent (consent for participation)
Human subjects (patients) were used for this study and patient recruitment was done in the vitreoretinal clinic of Sankara Nethralaya (Chennai-India) after obtaining informed consent from all the patients and controls to obtain 8 ml of blood sample.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance
1. We are the first to report a comparative analysis of HERPUD1 (rs2217332) gene association with PCV, AMD, and controls in the Indian population.
2. The genetic variant rs2217332 shows a strong association with PCV but not with AMD.
3. This data support that AMD and PCV have significant genetic differences, even though they share some common clinical features.
Limitation
1. Small number of samples were validated in both diseases
2. Indian population is heterogeneous and hence a large sample size may be good.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alagappan, L.P., Ramaswamy, Y., Sundaramoorthy, D.K. et al. Association of HERPUD1 genetic variant rs2217332 with age-related macular degeneration and polypoidal choroidal vasculopathy in an Indian cohort. Graefes Arch Clin Exp Ophthalmol 261, 1205–1212 (2023). https://doi.org/10.1007/s00417-022-05861-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05861-w