Abstract
Purpose
To determine the time to disease recurrence with long-acting injectable fluocinolone acetonide implant (FAi) for noninfectious intermediate, posterior, and panuveitis.
Methods
This was a retrospective study of patients with at least 12 months of follow-up who had completed a 2-year prospective, investigational new drug study with 0.18-mg FAi. Time to uveitis recurrence or cystoid macular edema (CME) occurrence was recorded.
Results
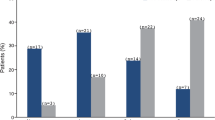
Twelve eyes from 12 participants (mean age 43 years, range 25–64 years) were included. Patients were followed for a mean of 34.2 months (range, 12.0–56.9 months) after completion of the prospective trial. Five eyes (42%) did not have a documented uveitis recurrence or CME occurrence. Five eyes (42%) had a uveitis recurrence with the mean time to recurrence 36.1 months (range, 22.8–61.1 months) after FAi implantation. Two eyes (16%) had CME alone, the mean time to occurrence 36.9 months (range 36.1–42.1 months). On Kaplan-Meier analysis, the estimated probability of remaining recurrence-free 36 months after FAi implantation was 0.67 (95% confidence interval, 0.34–0.86).
Conclusions
Data of study participants after completing a clinical trial suggest that the injectable FAi for noninfectious uveitis can provide control for 3 years on average. These long-term data support the use of FAi to control noninfectious uveitis.


Similar content being viewed by others
References
Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, Nussenblatt RB, Stiehm ER, Tessler H, Van Gelder RN, Whitcup SM, Yocum D (2000) Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 130:492–513. https://doi.org/10.1016/S0002-9394(00)00659-0
Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T, Fluocinolone Acetonide Uveitis Study Group (2006) Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 113:1020–1027. https://doi.org/10.1016/j.ophtha.2006.02.021
Kok H, Lau C, Maycock N, McCluskey P, Lightman S (2005) Outcome of intravitreal triamcinolone in uveitis. Ophthalmology 112:1916.e1–1916.e7. https://doi.org/10.1016/j.ophtha.2005.06.009
Multicenter Uveitis Steroid Treatment MUST Trial Research Group, Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Louis TA, Sugar EA, Thorne JE (2011) Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology 118:1916–1926. https://doi.org/10.1016/j.ophtha.2011.07.027
Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, Liesegang TL, Levy-Clarke GA, Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Jabs DA, Kempen JH (2014) Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology 121:2275–2286. https://doi.org/10.1016/j.ophtha.2014.05.021
Lowder C, Belfort R, Lightman S, Foster CS, Robinson MR, Schiffman RM, Li X-Y, Cui H, Whitcup SM, Ozurdex HURON Study Group (2011) Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol 129:545–553. https://doi.org/10.1001/archophthalmol.2010.339
Jaffe GJ, Foster CS, Pavesio CE, Paggiarino DA, Riedel GE (2019) Effect of an injectable Fluocinolone Acetonide insert on recurrence rates in chronic noninfectious uveitis affecting the posterior segment: twelve-month results. Ophthalmology 126:601–610. https://doi.org/10.1016/j.ophtha.2018.10.033
EyePoint Pharmaceuticals, Inc (2019) Yutiq (package insert). https://yutiq.com/downloads/YUTIQ-USPI-20181120.pdf. Accessed 14 May 2019
Jaffe GJ, Lin P, Keenan RT, Ashton P, Skalak C, Stinnett SS (2016) Injectable fluocinolone acetonide long-acting implant for noninfectious intermediate uveitis, posterior uveitis, and panuveitis: two-year results. Ophthalmology 123:1940–1948. https://doi.org/10.1016/j.ophtha.2016.05.025
Jaffe GJ (2019) Treatment of non-infectious uveitis that affects the posterior segment with a single intravitreal fluocinolone acetonide insert (FAi) – 3-year results. Invest Ophthalmol Vis Sci 60(9):3858
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 140:509–516. https://doi.org/10.1016/j.ajo.2005.03.057
Nussenblatt RB, Palestine AG, Chan CC, Roberge F (1985) Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 92:467–471. https://doi.org/10.1016/S0161-6420(85)34001-0
Goldstein DA, Godfrey DG, Hall A, Callanan DG, Jaffe GJ, Pearson PA, Usner DW, Comstock TL (2007) Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol 125:1478–1485. https://doi.org/10.1001/archopht.125.11.ecs70063
Acknowledgments
Wenlan Zhang, MD; Malav Joshi, MD; Sumit Sharma, MD; Scott Walter, MD: for participating in office visit assessments
Funding
The Heed Ophthalmic Foundation (CXC)
2018 Unrestricted Grant from Research to Prevent Blindness to Duke Eye Center (all)
Duke Eye Center P30 Core Grant from NIH/NEI (all)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CXC: none
CS: none
RTK: AbbVie, Inc. (speaker)
DSG: Alimera Sciences, Inc. (consultant), Clearside Biomedical, Inc. (consultant)
GJJ: EyePoint Pharmaceuticals, Inc. (consultant)
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, C.X., Skalak, C., Keenan, R.T. et al. Time to disease recurrence in noninfectious uveitis following long-acting injectable fluocinolone acetonide implant. Graefes Arch Clin Exp Ophthalmol 258, 1023–1030 (2020). https://doi.org/10.1007/s00417-020-04614-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04614-x