Abstract
Purpose
To analyse current off-label use of bevacizumab for wet age-related macular degeneration (AMD) in Europe.
Methods
The study was conducted as a combined survey and literature review. It included the 22 most populous countries in Europe. In each country, ophthalmologists with particular knowledge about off-label treatment responded to a questionnaire.
Results
Answers were obtained from twenty European countries. The off-label use of bevacizumab for wet AMD greatly differed between nations; the bevacizumab proportion varied from non-existent (0%) to very high (97%). There were also large disparities within single countries (e.g. 0–80%), which were attributable to differences in regional decision-making. Both governmental institutions and national ophthalmological societies expressed highly diverging opinions on the use of off-label treatment. Intravitreal administration of bevacizumab had been a matter of legal dispute in several countries. The question about responsibility for off-label therapy mainly remained unanswered.
Conclusions
There was a highly varying utilization of bevacizumab between European countries. Despite an intention of a consistent approach to medical regulations, Europe has not yet reached a professional or political consensus on the ophthalmic off-label use of bevacizumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
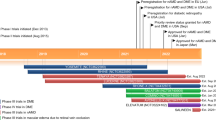
The term “off-label drug use” describes the utilization of an authorized medicinal product in a manner different from its marketing authorization (MA) [1]. It is a widespread medical practice and occurs for several reasons. First, drugs with MA for a certain purpose may not be available, which is typically the case for children, pregnant women, or patients with rare diseases. Secondly, an off-label drug is sometimes more effective than the registered therapy, such as rituximab for multiple sclerosis [2]. Finally, an inexpensive off-label drug can have comparable efficacy and safety to costlier therapy with MA. This is the case for bevacizumab (Avastin®; Roche, Basel, Switzerland). Although bevacizumab is only approved for systemic cancer therapy, it is used worldwide as an intravitreal treatment for retinal diseases, such as wet age-related macular degeneration (AMD), retinal vein occlusion (RVO), and diabetic macular oedema (DME). At the same time, ophthalmic off-label use of bevacizumab has caused great controversy because of the ethical, juridical, economic, and political issues surrounding its use [3].
Bevacizumab is a monoclonal, humanized, full-length antibody against vascular endothelial growth factor (VEGF). It was developed for use in breast-, lung- and gastrointestinal tract malignancies. However, many well-designed randomized controlled trials have also assessed the safety and effectiveness of bevacizumab in treatment of ophthalmic diseases, in particular wet AMD. The first major trials were the British inhibition of VEGF in age-related choroidal neovascularisation (IVAN) trial [4, 5] and the American comparison of age-related macular degeneration treatments trials (CATT) [6, 7]. The IVAN trial demonstrated that ranibizumab and bevacizumab had similar efficacy, and the frequency of serious adverse advents, such as arterial thrombotic events or hospital admission for heart failure, did not differ between the two drugs. The CATT study found that ranibizumab and bevacizumab had similar efficacy on visual acuity and that the rates of death, myocardial infarction, and stroke did not differ. Several other studies, such as BRAMD [8], MANTA [9], GEFAL [10], and LUCAS [11], have later confirmed that bevacizumab is non-inferior to ranibizumab for the treatment of wet AMD.
Modern Europe is dominated by the establishment of a political union, the European Union (EU), which shares common health objectives and focuses on giving equal access to modern and efficient healthcare for all citizens. The purpose of this study was to obtain a European overview of off-label use of bevacizumab for wet AMD in relation to professional and bureaucratic opinions in each country.
Methods
This study was conducted as a combined survey and literature review of legal documents, scientific publications, and available regulatory information regarding ophthalmic off-label use of bevacizumab for the treatment of wet AMD in Europe. The study included the 22 most populous countries in the EU and the European Free Trade Association. Literature was collected using PubMed, Google Scholar, and Google and search terms such as: “off-label”, “bevacizumab”, and “wet AMD”. Literature was also gathered from ophthalmologists with a particular knowledge of national use of Avastin. These were found by personal contacts, through their profiles on institutional webpages, or national ophthalmological societies. The experts were also e-mailed a questionnaire. The following questions were posed:
- 1.
What is the share of bevacizumab for wet AMD in your country today?
- 2.
Who pays the drug cost for the injection, i.e. public insurance/private insurance/out of pocket?
- 3.
What is the national ophthalmology society’s opinion on the ocular use of bevacizumab? Are there any policy documents about this issue?
- 4.
What is the national governmental authorities’ opinion on the ocular use of bevacizumab? Are there any policy documents addressing this topic?
- 5.
Who is responsible for harm caused by off-label bevacizumab use, i.e. doctor/national health insurance?
- 6.
Have there been any national legal disputes over ophthalmic use of bevacizumab?
All national experts were presented the final version of the manuscript and encouraged to review the description of their own country.
Results
With the exception of Slovenia and Greece, all countries responded to the survey. Results without a reference are survey answers from the expert ophthalmologists.
Austria
In Austria, approximately 90% of anti-VEGF treatment takes place in a public hospital setting and is refunded. There are no national recommendations regarding preferred use of anti-VEGF drugs. A nationwide study, MANTA, was conducted to compare the efficacy of bevacizumab and ranibizumab [9]. In hospitals, bevacizumab accounts for 20–60% of intravitreal injections, but aflibercept accounts for an increasing proportion. The treating physician is responsible for complications of off-label treatment. According to some retina specialists, the use of off-label drugs might be regarded illegal and remains an unresolved issue.
Belgium
In 2012, the Belgian Federal Agency for Drugs and Health products (FAGG/AFMPS) stated that bevacizumab should only be used to treat wet AMD in clinical trials and under the responsibility of a researcher. There is no reimbursement for ophthalmic use of bevacizumab, and the patient must pay an average of €50 for each injection. By comparison, the co-pay for ranibizumab and aflibercept is only €10. Nevertheless, bevacizumab has been commonly used as adjuvant therapy because of a wet AMD reimbursement limit for ranibizumab of 8 injections during the first year, 6 the second year, and 4 during the third year and beyond. Likewise, the aflibercept reimbursement for wet AMD has been limited to 17 injections over the first 3 years and 4 yearly injections thereafter. In 2016, following negotiations between the National Institute for Health and Invalidity Insurance (RIZIV/INAMI) and Novartis and Bayer, these limitations were discontinued, which again led to a decrease in bevacizumab use. At Turnhout General Hospital in northern Belgium, bevacizumab was used for 45–50% of intravitreal injections until 2015; in 2017 and 2018 the proportion dropped to 7%. National data on bevacizumab use do not exist.
Bulgaria
In Bulgaria, patients must pay about €150 for an off-label bevacizumab injection. On the other hand, aflibercept is partially covered by the health insurance system. Still, the patient’s co-payment is 20% higher for aflibercept than for bevacizumab. The Bulgarian Society of Ophthalmology recommends bevacizumab for treatment of wet AMD, and it has been used in 90% of all intravitreal therapy. However, it is estimated that the bevacizumab proportion decreased to about 70% following reimbursement of aflibercept from 2018. The Drug Safety Committee is responsible for side effects of drugs. There has been no national legal dispute over this matter.
Czech Republic
State health insurance does not cover the costs of intravitreal bevacizumab injections. Therefore only on-label treatment is possible in public hospitals, and ocular bevacizumab is used only in private clinics. The Czech Ophthalmic Society has no data on the frequency of bevacizumab use in the private sector. Legal regulations concerning off-label treatment in the Czech Republic are generally complicated. In the event of adverse effects following off-label bevacizumab treatment, the legal consequences may be serious, and the responsibility rests with the treating physician. The Czech Medical Chamber confirms this situation. The subject of off-label treatment, especially the legal consequences of vision loss or intraocular infections, has been subject to debate.
Denmark
Intravitreal bevacizumab is not administered in Denmark because neither the state nor the ophthalmological community is willing to take responsibility for the use of an off-label drug.
Finland
The Council for Choices in Health Care (COHERE) has stated that bevacizumab is an effective therapy for improving vision in wet AMD and that it is equal in efficacy and safety to ranibizumab and aflibercept. Bevacizumab is also included in the publicly funded healthcare service [12]. According to the opinion of the Finnish Expert Panel, there are no clinically significant differences in the efficacy and safety between the different anti-VEGF drugs in the treatment of wet AMD. The Finnish Medical Society, Duodecim, recommends bevacizumab as first-line therapy for wet AMD [13]. Out of 75,000 yearly injections for wet AMD, 80–85% are with bevacizumab. Aflibercept composes 10–15%, and one aflibercept vial is routinely divided into 3 syringes.
France
The national health insurance in France provides full coverage for all anti-VEGF treatment. Since 2015, the French Agency for the Safety of Health Products has supported ophthalmic use of bevacizumab by listing it as an allowed off-label drug. The recommendation was renewed in 2018 and made valid for another 3 years [14]. On the other hand, compounding of bevacizumab into prefilled syringes has been subject to very strict regulations, which practically makes bevacizumab difficult to obtain [15]. Fédération France Macula sees no clinically significant differences between bevacizumab and the authorized medicines but points out several unsolved problems regarding its off-label use [16]. The ophthalmic use of bevacizumab is very low and accounts for < 1% of all intravitreal injections. The doctor is responsible for all adverse events.
Novartis and Roche have challenged the off-label recommendations and claimed that the risk of infection is greater for bevacizumab than ranibizumab due to compounding. Nevertheless, in 2017 the French Administrative Supreme Court upheld its decision to support the ophthalmic use of bevacizumab [17].
Germany
Germany has a health insurance system that provides full coverage for all anti-VEGF treatment. As therapy freedom applies to physicians, it is legal to provide off-label treatment. However, health insurance coverage for off-label treatment is only possible if no approved alternative exists. The Federal Joint Committee sets the standards for reimbursement and publishes a list of acceptable off-label treatments, and bevacizumab for ocular use is not included [18]. Nevertheless, for many years, insurances have made contracts with medical care suppliers that offer a financial compensation when using bevacizumab, a legal situation that has been tolerated for more than a decade. In 2007, the German Ophthalmological Society stated that bevacizumab’s efficacy was similar to the approved drug, ranibizumab [19].
Bevacizumab accounted for nearly 35% of all intravitreal injections in 2017.
[20]. The treating physician is responsible for any adverse effect owing to off-label treatment and obliged to inform the patient about the medical and legal aspects [21]. In 2015, a legal dispute arose over the German company Apozyt for producing and selling bevacizumab and ranibizumab repacked into pre-filled syringes. Novartis initiated legal proceedings against Apozyt for unfair commercial competition. The matter was brought before the EU’s Court of Justice, and the court ruled that as long as modifications were not made to the product, a new marketing authorization was not required [22].
Hungary
The governmental authorities in Hungary oppose intravitreal off-label treatment with bevacizumab. Both the Hungarian Ophthalmological Society and the College of Ophthalmology follow this opinion. However, a few ophthalmic departments have applied for permission to use bevacizumab on an individual basis. The treating ophthalmologist is responsible for adverse events that occur after intravitreal injections.
Ireland
In Ireland, the public insurance pays for the majority of intravitreal injections. Some patients seek private clinics and payout of pocket. The two largest public retina services nearly exclusively use bevacizumab. Governmental authorities indirectly and directly encourage the use bevacizumab, whereas funding for registered drugs is discouraged by means of reimbursement obstacles. The ophthalmologic society’s opinion about off-label bevacizumab is broadly supportive. However, it expresses concern over doctors’ loss of autonomy and independence with regard to clinical decision-making. A “nonresponder” policy is promoted, i.e. bevacizumab is the first-line treatment, but switching to another drug is possible in the case of treatment resistance. For wet AMD, the share of bevacizumab is 70–80%. The ophthalmologist is responsible for adverse advents, and informed consent is advised. There have been no legal disputes over bevacizumab use.
Italy
Italy provides universal healthcare by a mixed public-private system. The public part is guaranteed by the National Health Service (SSN), which is organized under the Ministry of Health and administered regionally. The Italian Drugs Agency (AIFA) regulates the use of both approved drugs and off-label treatment.
If an approved drug is available, the physician is generally not allowed to prescribe an off-label alternative. Nevertheless, discrepancies are common at a local level. In 2012, AIFA dismissed SSN coverage of off-label ophthalmic use of bevacizumab, a decision that the Italian Ophthalmological Society (SOI) in turn disapproved. In 2014, AIFA reversed their 2012 dismission and allowed for ophthalmic off-label use of bevacizumab for both wet AMD and DME. Treatment is reimbursed by the SSN. Still, only authorized territorial pharmacies are allowed to compound bevacizumab for intravitreal use. In 2001, the Italian Ophthalmological Society (SOI) stated that bevacizumab’s efficacy was similar to that of ranibizumab [23]. Bevacizumab currently accounts for nearly 20% of the intravitreal injections for AMD. The Italian Competition Authority started investigative activities at the beginning of 2013 and finally imposed a €180 million fine on Novartis and Roche for “colluding to keep doctors from prescribing a relatively inexpensive drug” [24]. Off-label use of bevacizumab to treat wet AMD has received extensive media coverage and sparked a lively debate on how the present drug regulations may be revised [25].
Netherlands
In the Netherlands, a private health insurance is mandatory for all residents; the government decides the insurance coverage. There is a maximum yearly co-pay of about €550. The Dutch Ophthalmic Society’s AMD directive states that, based on cost differences, non-inferiority, and current insights on systemic side effects, bevacizumab is preferred to ranibizumab and aflibercept as first-line treatment [26]. On a national level, about 75–80% of intravitreal injections are with bevacizumab. The government has not opposed the use of bevacizumab. Instead, bevacizumab is officially reimbursed for treatment of wet AMD, DME, and RVO. The treating physician must inform the patient about the off-label treatment, and the Dutch Ophthalmic Society also recommends that the patient sign an informed consent. Patient’s organizations sued several ophthalmologists in 2008–2010 for the use of bevacizumab.
Norway
Anti-VEGF treatment in Norway includes all three drugs. In 2015, more than 60,000 intravitreal anti-VEGF injections were given, and the bevacizumab to aflibercept ratio was nearly 1:1 [27]. In most ophthalmic departments, bevacizumab is used as first-line drug, while aflibercept is used for treatment-resistant cases [28]. The Norwegian Ophthalmological Society suggests that bevacizumab should be considered as first-line treatment for wet AMD [29]. Likewise, the Norwegian Board of Health Supervision accepts off-label use of bevacizumab, provided that the treatment is justifiable and informed consent is obtained. Moreover, intravitreal bevacizumab treatment is officially reimbursed. The Norwegian System of Patient Injury Compensation reimburses all injuries originating from treatment in publicly funded clinics, regardless of off-label treatment. After two endophthalmitis outbreaks because of office-based splitting of anti-VEGF vials, pharmaceutical compounding of pre-filled syringes has become common; one aflibercept vial is routinely divided into 3 syringes [30]. In 2018, the controversy surrounding off-label use of bevacizumab and Novartis received considerable publicity in an article series in two of Norway’s major newspapers.
Poland
Although ophthalmic use of bevacizumab is regarded as off-label treatment in Poland, the Act on the medical profession states that the physician must follow the latest evidence-based practice. Since 2015, wet AMD patients must meet specific clinical requirements to receive reimbursement for aflibercept or ranibizumab treatment, and the institution must have signed a drug program contract with the National Health Fund (Narodowy Fundusz Zdrowia, NFZ) [31]. Bevacizumab is reimbursed for AMD patients who do not fulfil the aforementioned requirements. The NFZ also allows for bevacizumab treatment of RVO, DME, and myopic CNV. A large proportion of patients receiving bevacizumab are treated in private clinics. No national overview of various anti-VEGF drug proportions exists. Off-label treatment with bevacizumab has been subject to much debate in the Polish Ophthalmological Society (Polskie Towarzystwo Okulistyczne, PTO). The National Consultant for Ophthalmology has issued a position that bevacizumab’s effectiveness and safety are comparable to that of registered therapy. Consequently, bevacizumab can be considered a treatment option [32]. The PTO sent a letter to the Ministry of Health, asking for the official government authorities’ position on this matter, but no answer has been received so far.
Portugal
In Portugal, neither governmental authorities nor the Ophthalmological Society have officially expressed their opinion on intravitreal bevacizumab treatment. The use of intravitreal bevacizumab in public hospitals varies considerably. In some centres, bevacizumab is not used at all. In others, bevacizumab is used in nearly 80% of intravitreal injections and is typically used as first-line treatment. In private ophthalmic clinics, the bevacizumab proportion is about 70%, but the number is slowly decreasing.
Romania
Romania’s medical care system is predominantly public and financed by the National House of Insurances. Treatment in a public hospital is free of charge, provided that the National Insurance System approves the drugs and procedures. There are also private medical care options, for which the patient pays out of pocket; a private health insurance system does not exist. In 2007, leading authorities from the Retina Romanian Society (RRS) and the Romanian Medicines Agency (IRDA) collaborated to significantly increase the ophthalmic use of bevacizumab. In 2008, Roche issued a public letter to dissuade ophthalmic services from using bevacizumab, claiming it could put patients in danger. Subsequently, all public hospitals banned intravitreal use of bevacizumab, and in consequence, bevacizumab treatment now exclusively takes place in private clinics. Inexpensive treatment with bevacizumab has helped promote the private ophthalmic sector in Romania, and bevacizumab composes nearly 95–97% of intravitreal injections. However, the off-label therapy represents a jurisdictional ambiguity, and physicians treating patients with bevacizumab face a substantial legal risk. Nevertheless, within study protocols, bevacizumab has been proven to be an efficient and safe drug for wet AMD patients, both in short and medium term [33].
Spain
Medical expenses are publicly covered for patients treated in state hospitals but not in private practices. Approximately 90% of anti-VEGF treatment takes place in public hospitals. The Spanish Ophthalmic Society and the Spanish Vitreoretinal Society both advise that approved therapies for wet AMD should be used. Still, some regions with autonomous health decision-making capacity approve of bevacizumab as first-line treatment for wet AMD. The authorization does not apply to other diseases, such as DME, RVO, or myopic choroidal neovascularization (CNV). In the setting of insufficient response (strict criteria are lacking) to ranibizumab or aflibercept, however, bevacizumab may be used. It is estimated that bevacizumab composes about 13% of intravitreal injections nationwide [34]. In the absence of an institutional protocol, the treating physician is responsible for complications of off-label treatment.
Sweden
The Swedish healthcare system is government-funded and tax-financed. National principals and guidelines are established. Twenty-one county councils are responsible for delivering medical care. Each county has considerable leeway that explains substantial regional differences. In Sweden, bevacizumab has been used for wet AMD since the first CATT study was published in 2011. In 2016, bevacizumab was used for wet AMD in 10 out of 21 counties, and the bevacizumab share varied from 20 to 89%. There is a general trend towards less bevacizumab use in relatively rich and urbanized regions as compared with poorer and rural areas [35]. In 2011, the Swedish Ophthalmological Society stated that bevacizumab was a “satisfactory alternative” to ranibizumab [36]. The Dental and Pharmaceutical Benefits Agency, which decides if a drug should be reimbursed by the state, also endorses the use of off-label therapy [37]. On the other hand, the Swedish Medical Products Agency has stated that approved drugs should always be used if available [38]. In January 2018, the Swedish Drug Insurance, which is owned by the pharmaceutical industry, declared that it would not compensate for injuries associated with off-label treatment [39]. A similar statement from the National Patient Insurance followed [40]. Consequently, patients might not be compensated in the case of an adverse reaction attributable to intravitreal administration of bevacizumab.
Switzerland
In Switzerland, ranibizumab and aflibercept are fully reimbursed for treatment of wet AMD, DME, secondary CNV, and RVO. Accordingly, these drugs are routinely used in most clinics. Less than 0.5% of all injections are performed with bevacizumab. As there are only a few insurance agencies that refund intravitreal bevacizumab injections, it is rarely used.
United Kingdom
The government-run National Health Service (NHS) is the predominant provider of British healthcare. Patients do not pay for intravitreally administered drugs. All NHS trusts are obliged to use approved drugs. However, in January 2018, the National Institute for Health and Care Excellence concluded that there are no clinically significant differences between bevacizumab and its authorized analogues [41]. The decision was welcomed by the Royal College of Ophthalmologists, which had campaigned for off-label use of bevacizumab for wet AMD since 2012 [42, 43]. Data on the current use of bevacizumab are missing, but in 2015, bevacizumab constituted 3% of all intravitreal injections performed in NHS ophthalmic wards [44]. A possible turning point for ophthalmic use of bevacizumab came in September 2018, when an association of 12 clinical commissioning groups (CCGs, the NHS bodies responsible for the planning and commissioning of healthcare services for their local area) won the legal right to include bevacizumab as a routine treatment offer for wet AMD [45]. The cost of anti-VEGF treatment is not only considered an ophthalmologic issue but also a public health matter. The subject has received much attention in an article series in the British Medical Journal [46].
Discussion
All health systems within the EU proclaim to deliver patient-centred and individualized healthcare [47]. Still, our study results from 20 European countries showed that the member states had different approaches to these values, and several discrepancies in off-label use of bevacizumab for wet AMD was revealed (Table 1).
First, the bevacizumab proportion varied from large to non-existent. In several nations, there were also considerable internal disparities between different regions. Secondly, the national ophthalmological societies expressed highly diverging opinions, extending from positive to negative, or no opinion at all. Moreover, ophthalmological society statements favouring bevacizumab were inconsistently associated with both high and low national off-label use of the drug, and the same was true in regard to the opinion of governmental institutions. Thirdly, both drug companies and governmental authorities had raised legal disputes over ophthalmic use of bevacizumab. Finally, the question about responsibility for off-label drug use mainly remained unanswered. However, although it was frequently claimed that the doctor was accountable, no country could present a case in which a physician was held personally or economically liable for adverse events attributable to ophthalmic bevacizumab use. Taken together, the controversy over ophthalmic off-label use of bevacizumab remains remarkably unresolved within the world’s largest single market. In the United States, by contrast, there is a wider consensus, and a majority of American retina specialists use bevacizumab as the first-line drug [48].
Various drivers promote or prevent off-label use of medicinal products. The doctor-patient perspective, as expected, is a wish for therapy that is both safe and efficient. By contrast, the producers may see off-label pharmaceutical use of such a drug as a way to undermine fundamental principles of a regulatory framework. Medicine development takes a lot of time and money, and future investment initiatives could be halted if a therapeutic alternative bypasses normal legislative processes. In many cases, it is probably not a single driver but rather multiple factors that lead to a conclusion. The nature of the determinants might be complex, and they may also interact with each other. Still, multiple studies have proven bevacizumab to have comparable efficacy and safety to the registered anti-VEGF drugs, and there is also evidence that bevacizumab is the most cost-effective drug for wet AMD [49, 50]. In the end, the considerable European differences in ophthalmic bevacizumab use could mainly be a matter of principle and reflect differing attitudes towards off-label treatment itself. Moreover, in the perspective of a tax-funded healthcare system, an increasing expense inevitably necessitates decreased funding in another area. When more costly drugs become available, the society must ultimately prioritize between treatments and patient groups. In this regard, cutting the cost of healthcare by using an inexpensive off-label alternative becomes an attractive solution.
In conclusion, despite powerful political instruments, Europe has not yet reached a professional or political consensus on the ophthalmic off-label use of bevacizumab, despite a proven effectiveness. The varying utilization of off-label bevacizumab in European countries seems to contradict EU’s intention of a consistent approach to medical regulations [51]. Even if the results of clinical trials are the guideline for health policy throughout Europe, the drivers for off-label medication are complex and interact in an unknown manner. Regulatory levels, scientific societies and social discussions in media form the present system and makes it unlikely to establish a universal medical and pharmaceutical system within various European countries.
References
Aronson JK, Ferner RE (2017) Unlicensed and off-label uses of medicines: definitions and clarification of terminology. Br J Clin Pharmacol 83:2615–2625. https://doi.org/10.1111/bcp.13394
Salzer J, Svenningsson R, Alping P, Novakova L, Bjorck A, Fink K, Islam-Jakobsson P, Malmestrom C, Axelsson M, Vagberg M, Sundstrom P, Lycke J, Piehl F, Svenningsson A (2016) Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology 87:2074–2081. https://doi.org/10.1212/WNL.0000000000003331
Cohen D (2015) Why have UK doctors been deterred from prescribing Avastin? BMJ 350:h1654. https://doi.org/10.1136/bmj.h1654
Ivan Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 119:1399–1411. https://doi.org/10.1016/j.ophtha.2012.04.015
Ivan Study Investigators, Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, Reeves BC (2013) Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 382:1258–1267. https://doi.org/10.1016/S0140-6736(13)61501-9
CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364:1897–1908. https://doi.org/10.1056/NEJMoa1102673
CATT Research Group, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 119:1388–1398. https://doi.org/10.1016/j.ophtha.2012.03.053
Schauwvlieghe AM, Dijkman G, Hooymans JM, Verbraak FD, Hoyng CB, Dijkgraaf MG, Peto T, Vingerling JR, Schlingemann RO (2016) Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age-related macular degeneration. The BRAMD Study. PloS one 11:e0153052. https://doi.org/10.1371/journal.pone.0153052
Krebs I, Schmetterer L, Boltz A, Told R, Vecsei-Marlovits V, Egger S, Schonherr U, Haas A, Ansari-Shahrezaei S, Binder S, Group MR (2013) A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol 97:266–271. https://doi.org/10.1136/bjophthalmol-2012-302391
Kodjikian L, Souied EH, Mimoun G, Mauget-Faysse M, Behar-Cohen F, Decullier E, Huot L, Aulagner G, Group GS (2013) Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology 120:2300–2309. https://doi.org/10.1016/j.ophtha.2013.06.020
Berg K, Pedersen TR, Sandvik L, Bragadottir R (2015) Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 122:146–152. https://doi.org/10.1016/j.ophtha.2014.07.041
Council for Choices in Health Care in Finland (2015) The treatment of wet age-related macular degeneration with bevacizumab injection in the eye belongs to the publicly funded service choices in health care in Finland. https://palveluvalikoima.fi/en/recommendations. Accessed 2019-07-09
Tuuminen R, Sipila R, Komulainen J, Saarela V, Kaarniranta K, Tuulonen A (2019) The first ophthalmic Choosing Wisely recommendations in Finland for glaucoma and wet age-related macular degeneration. Acta Ophthalmol. https://doi.org/10.1111/aos.14031
L’Agence nationale de sécurité du médicament et des produits de santé Liste des spécialités faisant l'objet d'une RTU. https://www.ansm.sante.fr/Activites/Recommandations-Temporaires-d-Utilisation-RTU/Liste-des-specialites-faisant-actuellement-l-objet-d-une-RTU/(offset)/1. Accessed 2018-12-12
Tick S, Cornut PL, De Bats F, Wolf B, Souied EH, Cohen SY (2018) Update from France Macula Federation: Treatment of Wet AMD. J Fr Ophtalmol 41:862–867. https://doi.org/10.1016/j.jfo.2018.06.002
Cohen SY, Souied E (2015) Analyse de la littérature et synthèse : Bevacizumab vs autres anti-VEGF. http://www.ffmacula.fr/sites/default/files/pdf/analyse_de_litterature_avastin.pdf. Accessed 2019-04-30
Conseil d'État (2017) N° 392459. https://www.legifrance.gouv.fr/affichJuriAdmin.do?oldAction=rechJuriAdmin&idTexte=CETATEXT000034081845&fastReqId=1568863364&fastPos=12. Accessed 2019-07-18
Bundesausschuss G (2018) Anlage IV zum Abschnitt K der Arzneimittel-Richtlinie. https://www.g-ba.de/informationen/richtlinien/anlage/15/. Accessed 2018-12-27
German Ophthalmological Society & Retinological Society & Association of Ophthalmologists in Germany (2007) Statement of the German Ophthalmological Society, the Retinological Society and the Association of Ophthalmologists in Germany on current therapeutic options in neovascular age-related macular degeneration https://www.dog.org/wp-content/uploads/2009/08/DOG_Statement_AMDTherapy.pdf. Accessed 2018-10-28
Martin W, Burkhard Dick H, Scharrer A, Schayan K, Reinhard T (2018) Umfrage von BDOC, BVA, DGII und DOG zur ambulanten und stationären Intraokularchirurgie: Ergebnisse für das Jahr 2017. OPHTHALMO-CHIRURGIE 30:255–266
den Exter A, Foldes ME (2014) Casebook on European Union Health Law. Maklu Publishers
Court of Justice of the European Union (2013) Judgment of the Court C-535/11
Società Oftalmologica Italiana (2012) Documento cts soi su Avastin. https://www.sedesoi.com/leggi_news.php?id=1129&anno2=2012. Accessed 2019-07-09
Cohen D (2014) Roche and Novartis colluded over wet AMD drugs, says Italian regulator. BMJ 348:g2006. https://doi.org/10.1136/bmj.g2006
Messori A (2014) Avastin-Lucentis: off-label and surroundings. Recenti Prog Med 105:137–140. https://doi.org/10.1701/1459.16117
Nederlands Oogheelkundig Gezelschap (2014) Richtlijn Leeftijdgebonden Maculadegeneratie. https://maculavereniging.nl/wp-content/uploads/2015/04/Richtlijn-LMD-GEAUTORISEERDE-VERSIE-270314.pdf. Accessed 2019-09-19
Kristiansen IS, Haugli Braten R, Jorstad OK, Moe MC, Saether EM (2019) Intravitreal therapy for retinal diseases in Norway 2011-2015. Acta Ophthalmol. https://doi.org/10.1111/aos.14262
Jorstad OK, Faber RT, Moe MC (2017) Two-year functional and anatomical results after converting treatment resistant eyes with exudative age-related macular degeneration to aflibercept in accordance with a treat and extend protocol. Acta Ophthalmol 95:460–463. https://doi.org/10.1111/aos.13480
Norsk oftamologisk forening (2017) Nasjonal kvalitetshåndbok for oftalmologi. https://www.helsebiblioteket.no/retningslinjer/oftalmologi/forord. Accessed 2019-09-19
Sivertsen MS, Jorstad OK, Grevys A, Foss S, Moe MC, Andersen JT (2018) Pharmaceutical compounding of aflibercept in prefilled syringes does not affect structural integrity, stability or VEGF and Fc binding properties. Sci Rep 8:2101. https://doi.org/10.1038/s41598-018-20525-8
National Health Fund (2017) Treatment of neovascular (wet) form of macular degeneration (AMD) (ICD-10 H35.3). https://www.gov.pl/documents/292343/436711/b.70.-nowy-od-01.2017.docx/0761e567-61ce-808b-eeed-0f202c0d6f46. Accessed 2019-11-26
PTO (2017) Position of the National Consultant on Ophthalmology on the use of intravitreal injections of bevacizumab (Avastin, Roche) in the treatment of retinal disorders. http://adst.mp.pl/s/www/okulistyka/Avastin2017.pdf. Accessed 2019-07-19
Stanca HT, Stanca S, Tabacaru B, Boruga M, Balta F (2019) Bevacizumab in Wet AMD treatment: A tribute to the thirteen years of experience from the beginning of the anti-VEGF era in Romania. Exp Ther Med 18:4993–5000
The American Society of Retina Specialists (2018) Global Trends in Retina Survey. https://www.asrs.org/sections/international/global-trends-in-retina. Accessed 2019-07-18
Styrgruppen för Svenska Makularegistret (2017) Årsrapport för allmänheten 2016 Svenska Makularegistret. http://rcsyd.se/makulareg/wp-content/uploads/sites/2/2018/02/%C3%85rsrapportSMRallm%C3%A4nheten.pdf. Accessed 2018-12-14
Seregard S (2011) Avastin eller Lucentis? En fortsättning. Ett Ögonblick 3
Tandvårds- och läkemedelsförmånsverket (The Dental and Pharaceutical Benefits Agency) (2017) Ändring i Tandvårds- och läkemedelsförmånsverkets allmänna råd (TLVAR 2003:2) om ekonomiska utvärderingar (Change in The Dental and Pharaceutical Benefits Agency sommon advice on financial evaluations). https://www.tlv.se/download/18.7e3d365215ec82458645a7/1510316403483/TLVAR_2015_1.pdf. Accessed 2019-11-26
Läkemedelsverket (2016) Läkemedelsverkets syn på användning av läkemedel utanför det regulatoriska godkännandet. Information från Läkemedelsverket 27:13–14
Läkemedelsförsäkringen (2018) Åtagande att utge ersättning för läkemedelsskada. https://lff.se/wp-content/uploads/2014/02/Atagande-2018-01-01.-final.pdf Accessed
Hake C-M (2018) Prislapp klar för off label-försäkring. https://www.dagensmedicin.se/artiklar/2018/10/24/prislapp-klar-for-off-label-forsakring/. Accessed 2019-07-09
National institute for health and care excellens (2018) NICE Guidance Age-related macular degeneration. https://www.nice.org.uk/guidance/NG82. Accessed 2019-07-09
The Royal College of Ophthalmologists (2018) New NICE Age Related Macular Degeneration guidance supports potential cost savings for the NHS. https://www.rcophth.ac.uk/2018/01/new-nice-age-related-macular-degeneration-guidance-supports-potential-cost-savings-for-the-nhs/. Accessed 2019-07-09
The Royal College of Ophthalmologists (2018) The Royal College of Ophthalmologists is delighted at landmark ruling in favour of the use of Avastin for wet AMD. https://www.rcophth.ac.uk/2018/09/the-royal-college-of-ophthalmologists-is-delighted-that-the-high-court-has-found-in-favour-of-the-use-of-avastin-for-wet-amd/. Accessed 2019-07-09
Shalaby AK, Lewis K, Bush K, Meredith PR, Di Simplicio S, Lockwood AJ (2016) Licence to save: a UK survey of anti-VEGF use for the eye in 2015. Eye 30:1404–1406. https://doi.org/10.1038/eye.2016.154
Cohen D (2018) CCGs win right to offer patients Avastin for wet AMD. Bmj 362:k4035. https://doi.org/10.1136/bmj.k4035
Parikh R, Pirakitikulr N, Chhablani J, Sakurada Y, Singh RP, Modi YS (2019) A Multinational Comparison of Anti-Vascular Endothelial Growth Factor Use: The United States, the United Kingdom, and Asia-Pacific. Ophthalmol Retina 3:16–26. https://doi.org/10.1016/j.oret.2018.08.002
THE COUNCIL OF THE EUROPEAN UNION (2006) Council Conclusions on Common values and principles in European Union Health Systems (2006/C 146/01)Official Journal of the European Union
American Society of Retina Specialists Preferences and trends Survey 2018. https://www.asrs.org/content/documents/2018-global-trends-in-retina-survey-highlights-website.pdf. Accessed 2019-06-21
Elshout M, Webers CAB, van der Reis MI, Schouten J (2018) A systematic review on the quality, validity and usefulness of current cost-effectiveness studies for treatments of neovascular age-related macular degeneration. Acta Ophthalmol 96:770–778. https://doi.org/10.1111/aos.13824
Low A, Faridi A, Bhavsar KV, Cockerham GC, Freeman M, Fu R, Paynter R, Kondo K, Kansagara D (2019) Comparative effectiveness and harms of intravitreal antivascular endothelial growth factor agents for three retinal conditions: a systematic review and meta-analysis. Br J Ophthalmol 103:442–451. https://doi.org/10.1136/bjophthalmol-2018-312691
NIVA RIVM EPHA, Weda M, Hoebert J, Vervloet M, Puigmarti CM, Damen N, Marchange S, Langedijk J, Lisman J, Dijk Lv (2017) Study on off-label use of medicinal products in the European Union European Union
Acknowledgements
Assistance provided by the following national authorities in retinal diseases is greatly appreciated. Austria: Susanne Binder MD PhD. Belgium: Werner Dirven MD. Bulgaria: Christina Vidinova MD. Czech Republic: Sarka Pitrova MD PhD.
Denmark: Toke Bek MD PhD. Finland: Kai Kaarniranta MD PhD.
France: Salomon Yves Cohen MD PhD, Catherine Creuzot, MD, PhD, Laurent Kodjikian MD PhD. Germany: Horst Helbig MD PhD. Hungary: Goran Petrovski MD PhD. Andrea Facskó MD PhD. Ireland: David Keegan MD PhD. Italy: Francesco Bandello MD PhD, Enrico Borrelli MD, Giuseppe Querques MD PhD. Netherlands: Tom Missotten MD, Reinier O. Schlingemann MD PhD. Norway: Morten Carstens Moe MD PhD, Karina Birgitta Berg MD PhD. Portugal: Angela Carneiro MD PhD. Romania: Horia T. Stanca MD PhD. Spain: José Maria Ruiz Moreno MD PhD, Jose Garcia-Arumi MD PhD, María Moreno-Lopez MD. Switzerland: Sebastian Wolf MD PhD. United Kingdom: John Lawrensson MSc PhD, Usha Chakravarthy MD, PhD.
Funding
Open access funding provided by Lund University. This study was funded by Futurum - Akademin för vård och hälsa Region Jönköpings län (grant number FUTURUM-808791).
Author information
Authors and Affiliations
Contributions
ØKJ is member of a Bayer advisory board and has received lecture fees from Bayer and Allergan.
Corresponding author
Ethics declarations
Conflict of interest
The other authors declare that they have no conflict of interest in this research.
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors. This study was funded by Futurum - Akademin för vård och hälsa Region Jönköpings län (grant number FUTURUM-808791).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bro, T., Derebecka, M., Jørstad, Ø.K. et al. Off-label use of bevacizumab for wet age-related macular degeneration in Europe. Graefes Arch Clin Exp Ophthalmol 258, 503–511 (2020). https://doi.org/10.1007/s00417-019-04569-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04569-8