Abstract
Purpose
Real-life studies on long-term functional outcome of anti-VEGF treatment for wet age-related macular degeneration (wAMD) are limited. We therefore assessed the 10-year outcomes in our patients.
Methods
In this retrospective study, all patients with newly diagnosed wAMD that had received minimally three intravitreal injections between 2007 and 2012 and a follow-up of ≥48 months were included. Primary outcome measure was the evolution of best-corrected visual acuity (BCVA) over time. For qualitative, quantitative and longitudinal data, Pearson's chi2 test, the Mann–Whitney U-test and Wilcoxon’s signed-rank test were applied at a significance level of p < 0.05.
Results
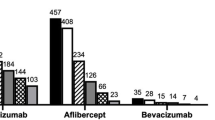
Of 267 eyes (219 patients) with newly diagnosed wAMD treated during this period, 104 eyes (104 patients) had been followed for at least 48 months and were included. Fifty-nine eyes (57.8%) after 7 years were still under active treatment, 29 eyes (25.0%) had interrupted treatment [mean follow-up 7.5 years (4.0–10.1; SD 1.6)], whereas 16 patients had died. BCVA stabilized at −7.3 to −11.9 letters after 3–10 years of follow-up with a mean of 2.8 injections (median; 3.0, SD 1.0; 1–5) and 5.1 visits per year. In two thirds of eyes, treatment was switched to aflibercept or corticosteroid combinations without bearing on functional outcomes. Thirty-seven percent (37%) of eyes maintained driving vision for up to 10 years.
Conclusions
Beyond 3 years of treatment, functional stability was maintained for up to 10 years. Further improvement of long-term outcomes might have required a more intensive treatment in the early phase.






Similar content being viewed by others
References
Holz FG, Amoaku W, Donate J, SUSTAIN Study Group et al (2011) Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 118:663–671. https://doi.org/10.1016/j.ophtha.2010.12.019
Martin DF, Maguiere MG, Fine SL, CATT Research Group et al (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364:1897–1908. https://doi.org/10.1056/NEJMoa1102673
Chakravarthy U, Harding SP, Rogers CA, IVAN study investigators et al (2013) Alternative treatments to inhibit VEGF in age-related choroidal neovascularization: 2-year findings of the IVAN randomized controlled trial. Lancet 382:1258–1267. https://doi.org/10.1016/S0140-6736(13)61501-9
Quaggin SE (2012) Turning a blind eye to anti-VEGF toxicities. J Clin Invest 122:3849–3851. https://doi.org/10.1172/JCI65509
Rofagha S, Bhisitkul RB, Boyer DS, SEVEN-UP Study Group et al (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 120:2292–2299. https://doi.org/10.1016/j.ophtha.2013.03.046
Finger RP, Wickremasinghe SS, Baird PN et al (2014) Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol 59:1–18. https://doi.org/10.1016/j.survophthal.2013.03.009
Muether PS, Hoerster R, Hermann MM et al (2013) Long-term effects of ranibizumab treatment delay in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 251:453–458. https://doi.org/10.1007/s00417-012-2038-0
Michels S, Rosenfeld PJ, Puliafito CA et al (2005) Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 112:1035–1047. https://doi.org/10.1016/j.ophtha.2005.0.007
Rosenfeld PJ, Moshfeghi AA, Puliafito CA (2005) Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 36:331–335. https://doi.org/10.3928/1542-8877-20050701-14
Heier JS, Antoszyk AN, Pavan PR et al (2006) Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology 113:633.e1-4. https://doi.org/10.1016/j.ophtha.2005.10.052
Kaiser PK, Brown DM, Zhang K et al (2007) Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol 144:850–857. https://doi.org/10.1016/j.ajo.2007.08.012
Rosenfeld PJ, Brown DM, Heier JS, MARINA Study Group et al (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. https://doi.org/10.1056/NEJMoa054481
Heier JS, Brown DM, Chong V et al (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119(12):2537–2548. https://doi.org/10.1016/j.ophtha.2012.09.006
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF et al (2014) Intravitreal Aflibercept injection for neovascular age-related macular degeneration. Ophthalmology 121:193–201. https://doi.org/10.1016/j.ophtha.2013.08.011
Bhisitkul RB, Mendes TS, Rofagha S et al (2015) Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol 159:915–24.e2. https://doi.org/10.1016/j.ajo.01.032
Bhisitkul RB, Desai SJ, Boyer DS et al (2016) Fellow eye comparisons for 7-year outcomes in Ranibizumab-treated AMD subjects from ANCHOR, MARINA, and HORIZON (SEVEN-UP study). Ophthalmology 123:1269–1277. https://doi.org/10.1016/j.ophtha.2016.01.033
Garweg JG (2016) Atrophy of the macula in the context of its wet, age-related degeneration: an inescapable consequence of anti-VEGF therapy? Ophthalmologe 113:1036–1045. https://doi.org/10.1007/s00347-016-0306-9
Holz FG, Tadayoni R, Beatty S et al (2015) Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 99:220–226. https://doi.org/10.1136/bjophthalmol-2014-305327
Munk MR, Ceklic L, Ebneter A et al (2016) Macular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol 94:e757–e764. https://doi.org/10.1111/aos.13157
Boulanger-Scemama E, Sayag D, Ha Chau Tran T et al (2016) Ranibizumab and exudative age-related macular degeneration: 5-year multicentric functional and anatomical results in real-life practice. J Fr Ophtalmol 39:668–674. https://doi.org/10.1016/j.jfo.2016.06.001
Maguire MG, Martin DF, Ying GS, Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group et al (2016) Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology 123:1751–1761. https://doi.org/10.1016/j.ophtha.2016.03.045
Borooah S, Jeganathan VS, Ambrecht AM et al (2015) Long-term visual outcomes of intravitreal ranibizumab treatment for wet age-related macular degeneration and effect on blindness rates in south-east Scotland. Eye (Lond) 29:1156–1161. https://doi.org/10.1038/eye.2015.83
Boulanger-Scemama E, Querques G, About F et al (2015) Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol 38:620–627. https://doi.org/10.1016/j.jfo.2014.11.015
Gillies MC, Campain A, Barthelmes D, Fight Retinal Blindness Study Group et al (2015) Long-term outcomes of treatment of Neovascular age-related macular degeneration: data from an observational study. Ophthalmology 122:1837–1845. https://doi.org/10.1016/j.ophtha.2015.05.010
Peden MC, Suñer IJ, Hammer ME et al (2015) Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology 122:803–808. https://doi.org/10.1016/j.ophtha.2014.11.018
Holz FG, Tadayoni R, Beatty S et al (2016) Determinants of visual acuity outcomes in eyes with neovascular AMD treated with anti-VEGF agents: an instrumental variable analysis of the AURA study. Eye (Lond) 30:1063–1071. https://doi.org/10.1038/eye.2016.90
Holz FG, Tadayoni R, Beatty S et al (2016) Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol 100:1623–1628. https://doi.org/10.1136/bjophthalmol-2015-308166
Razi F, Haq A, Tonne P et al (2016) Three-year follow-up of ranibizumab treatment of wet age-related macular degeneration: influence of baseline visual acuity and injection frequency on visual outcomes. Clin Ophthalmol 10:313–319. https://doi.org/10.2147/OPTH.S97775
Rasmussen A, Sander B (2014) Long-term longitudinal study of patients treated with ranibizumab for neovascular age-related macular degeneration. Curr Opin Ophthalmol 25:158–163. https://doi.org/10.1097/ICU.0000000000000050
Kuehlewein L, Dustin L, Sagong M et al (2016) Predictors of macular atrophy detected by Fundus autofluorescence in patients with Neovascular age-related macular degeneration after long-term Ranibizumab treatment. Ophthalmic Surg Lasers Imaging Retina 47:224–231. https://doi.org/10.3928/23258160-20160229-04
Chew EY, Clemons TE, Agrón E, Age-Related Eye Disease Study Research Group et al (2014) Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol 132:272–277. https://doi.org/10.1001/jamaophthalmol.2013.6636
Amstutz CA, Fleischhauer J, Zweifel S et al (2015) Long-term outcome in patients with Intravitreal anti-VEGF therapy for Exudative AMD. Klin Monatsbl Augenheilkd 232:533–537. https://doi.org/10.1055/s-0035-1545673
Acknowledgements
None of the authors announces any conflict of interest in the context of the data presented herein.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JGG advises several pharmaceutical companies (Alcon, Allergan, Bayer, Novartis) and participates in a number of international, multicentre clinical studies that are sponsored by a few of these (Novartis, Bayer) in the fields of AMD and diabetic retinopathy. These activities had no bearing on the study that gave rise to the submitted article, for which JGG received neither direct nor indirect financial support; nor has he conflicts of interest with any of the presented data. The other two authors have no potential conflicts of interest.
Financial disclosure
This study did not receive any external funding.
Ethical approval
Though formal consent is not required for this type of retrospective study, ethical approval was received by the Institutional Ethics Board (Kantonale Ethikkommission Bern, University of Bern, registration number KEK 99/15).
Rights and permissions
About this article
Cite this article
Garweg, J.G., Zirpel, J.J., Gerhardt, C. et al. The fate of eyes with wet AMD beyond four years of anti-VEGF therapy. Graefes Arch Clin Exp Ophthalmol 256, 823–831 (2018). https://doi.org/10.1007/s00417-018-3907-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-3907-y